当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of 1,3-Enynes by Iron-Catalyzed Propargylic C–H Functionalization: An Alkyne Analogue for the Eschenmoser Methenylation
Organic Letters ( IF 4.9 ) Pub Date : 2024-04-11 , DOI: 10.1021/acs.orglett.4c00696
Shalini Dey 1 , Aaron D Charlack 1 , Austin C Durham 1 , Jin Zhu 1 , Yidong Wang 1, 2 , Yi-Ming Wang 1
Organic Letters ( IF 4.9 ) Pub Date : 2024-04-11 , DOI: 10.1021/acs.orglett.4c00696
Shalini Dey 1 , Aaron D Charlack 1 , Austin C Durham 1 , Jin Zhu 1 , Yidong Wang 1, 2 , Yi-Ming Wang 1
Affiliation
|
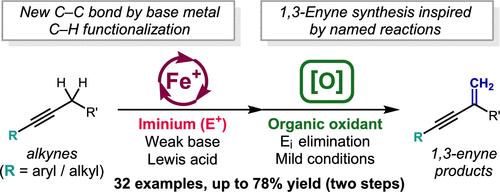
A two-step protocol for the conversion of alkyl-substituted alkynes to 1,3-enynes is reported. In this α-methenylation process, an iron-catalyzed propargylic C–H functionalization delivers tetramethylpiperidine-derived homopropargylic amines which undergo facile Cope elimination upon N-oxidation to afford the enyne products. A range of aryl alkyl and dialkyl acetylenes were found to be suitable substrates for this process, which constitutes an alkyne analogue for the Eschenmoser methenylation of carbonyl derivatives. In addition, a new bench-stable precatalyst for iron-catalyzed propargylic C–H functionalization is reported.
中文翻译:

铁催化炔丙 C-H 官能化合成 1,3-烯炔:用于 Eschenmoser 甲基化的炔烃类似物
报道了将烷基取代的炔烃转化为 1,3-烯炔的两步方案。在这个 α-亚甲基化过程中,铁催化的炔丙 C-H 官能化产生四甲基哌啶衍生的均炔丙胺,这些胺在 N-氧化后容易发生 Cope 消除,得到烯炔产物。一系列芳基烷基和二烷基乙炔被发现是该过程的合适底物,其构成了用于羰基衍生物的埃舍莫瑟甲基化的炔类似物。此外,还报道了一种用于铁催化炔丙 C-H 官能化的新型台式稳定预催化剂。
更新日期:2024-04-11
中文翻译:

铁催化炔丙 C-H 官能化合成 1,3-烯炔:用于 Eschenmoser 甲基化的炔烃类似物
报道了将烷基取代的炔烃转化为 1,3-烯炔的两步方案。在这个 α-亚甲基化过程中,铁催化的炔丙 C-H 官能化产生四甲基哌啶衍生的均炔丙胺,这些胺在 N-氧化后容易发生 Cope 消除,得到烯炔产物。一系列芳基烷基和二烷基乙炔被发现是该过程的合适底物,其构成了用于羰基衍生物的埃舍莫瑟甲基化的炔类似物。此外,还报道了一种用于铁催化炔丙 C-H 官能化的新型台式稳定预催化剂。































 京公网安备 11010802027423号
京公网安备 11010802027423号