当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Total Synthesis of (±)-Oxacyclododecindione
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2024-04-10 , DOI: 10.1021/acs.joc.4c00333
Kevin Seipp 1 , Vincent Grölz 1 , Hagen Glass 1 , Elisabeth Quraishi 1 , Nina Vierengel 1 , Till Opatz 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2024-04-10 , DOI: 10.1021/acs.joc.4c00333
Kevin Seipp 1 , Vincent Grölz 1 , Hagen Glass 1 , Elisabeth Quraishi 1 , Nina Vierengel 1 , Till Opatz 1
Affiliation
|
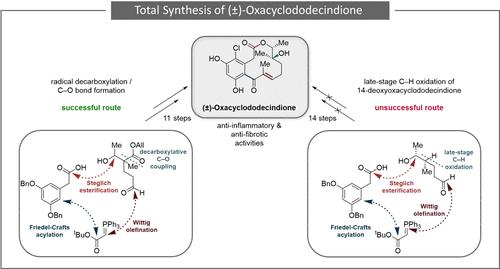
Racemic total synthesis of the natural product oxacyclododecindione, isolated in 2008 as the first member of the oxacyclododecindione family, is reported. Studies toward this molecule commenced with a biomimetic late-stage C–H oxidation starting from 14-deoxyoxacyclododecindione as a known precursor. This provided insights into the reactivity of the macrolactone class but did not permit the synthesis of the target natural product. Based on these results, a synthetic strategy through intramolecular Friedel–Crafts acylation combined with Barton decarboxylation to introduce the tertiary alcohol, a major challenge in previous synthetic efforts, was envisioned. This resulted in an 11-step racemic total synthesis of (±)-oxacyclododecindione, renowned for its potent anti-inflammatory and antifibrotic activities.
中文翻译:

(±)-氧杂环十二烷二酮的全合成
据报道,天然产物 oxacyclododecindione 的外消旋全合成于 2008 年分离,是 oxacyclododecindione 家族的第一个成员。对该分子的研究始于 14-脱氧环十二烷二酮作为已知前体的仿生晚期 C-H 氧化。这提供了对大内酯类反应性的见解,但不允许合成目标天然产物。基于这些结果,设想了一种通过分子内 Friedel-Crafts 酰化结合 Barton 脱羧引入叔醇的合成策略,这是以前合成工作中的一个主要挑战。这导致了 (±)-氧氧环十二烷二酮的 11 步外消旋全合成,该合成以其强大的抗炎和抗纤维化活性而闻名。
更新日期:2024-04-10
中文翻译:

(±)-氧杂环十二烷二酮的全合成
据报道,天然产物 oxacyclododecindione 的外消旋全合成于 2008 年分离,是 oxacyclododecindione 家族的第一个成员。对该分子的研究始于 14-脱氧环十二烷二酮作为已知前体的仿生晚期 C-H 氧化。这提供了对大内酯类反应性的见解,但不允许合成目标天然产物。基于这些结果,设想了一种通过分子内 Friedel-Crafts 酰化结合 Barton 脱羧引入叔醇的合成策略,这是以前合成工作中的一个主要挑战。这导致了 (±)-氧氧环十二烷二酮的 11 步外消旋全合成,该合成以其强大的抗炎和抗纤维化活性而闻名。

































 京公网安备 11010802027423号
京公网安备 11010802027423号