当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Asymmetric Sulfonylation from a Reaction of Cyclopropan-1-ol, Sulfur Dioxide, and 1-(Alkynyl)naphthalen-2-ol
Organic Letters ( IF 4.9 ) Pub Date : 2024-04-10 , DOI: 10.1021/acs.orglett.4c01011 Chun Zhang 1 , Shengqing Ye 1 , Jie Wu 1, 2, 3, 4
Organic Letters ( IF 4.9 ) Pub Date : 2024-04-10 , DOI: 10.1021/acs.orglett.4c01011 Chun Zhang 1 , Shengqing Ye 1 , Jie Wu 1, 2, 3, 4
Affiliation
|
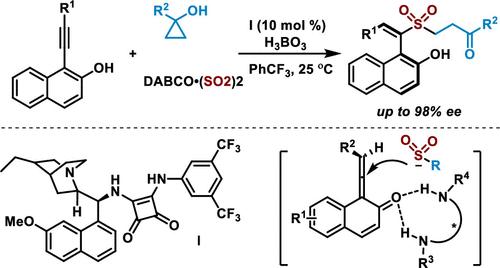
Asymmetric sulfonylation from a reaction of cyclopropan-1-ol, sulfur dioxide, and 1-(alkynyl)naphthalen-2-ol in the presence of a catalytic amount of organocatalyst at room temperature is developed. Axially chiral (S)-(E)-1-(1-(alkylsulfonyl)-2-arylvinyl)naphthalen-2-ols are generated in moderate to good yields with excellent enantioselectivity and regioselectivity under mild conditions. During this transformation, γ-keto sulfinate generated in situ from cyclopropan-1-ol and sulfur dioxide acts as the key intermediate.
中文翻译:

1-环丙醇、二氧化硫和 1-(炔基)萘-2-醇反应的不对称磺酰化
开发了在催化量的有机催化剂存在下在室温下由环丙-1-醇、二氧化硫和 1-(炔基)萘-2-醇反应进行的不对称磺酰化。轴向手性 ( S )-( E )-1-(1-(烷基磺酰基)-2-芳基乙烯基)萘-2-醇在温和条件下以中等至良好的产率生成,具有优异的对映选择性和区域选择性。在此转化过程中,由环丙醇和二氧化硫原位生成的γ-酮亚磺酸盐充当关键中间体。
更新日期:2024-04-10
中文翻译:

1-环丙醇、二氧化硫和 1-(炔基)萘-2-醇反应的不对称磺酰化
开发了在催化量的有机催化剂存在下在室温下由环丙-1-醇、二氧化硫和 1-(炔基)萘-2-醇反应进行的不对称磺酰化。轴向手性 ( S )-( E )-1-(1-(烷基磺酰基)-2-芳基乙烯基)萘-2-醇在温和条件下以中等至良好的产率生成,具有优异的对映选择性和区域选择性。在此转化过程中,由环丙醇和二氧化硫原位生成的γ-酮亚磺酸盐充当关键中间体。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号