当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of 1-(Halo)alkyl-3-heteroaryl Bicyclo[1.1.1]pentanes Enabled by a Photocatalytic Minisci-type Multicomponent Reaction
ACS Catalysis ( IF 11.3 ) Pub Date : 2024-04-10 , DOI: 10.1021/acscatal.4c00740 Jiashun Zhu 1 , Yu Hong 1 , Yuxin Wang 1 , Yirui Guo 1 , Yuru Zhang 1 , Zhigang Ni 1 , Wanmei Li 1 , Jun Xu 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2024-04-10 , DOI: 10.1021/acscatal.4c00740 Jiashun Zhu 1 , Yu Hong 1 , Yuxin Wang 1 , Yirui Guo 1 , Yuru Zhang 1 , Zhigang Ni 1 , Wanmei Li 1 , Jun Xu 1
Affiliation
|
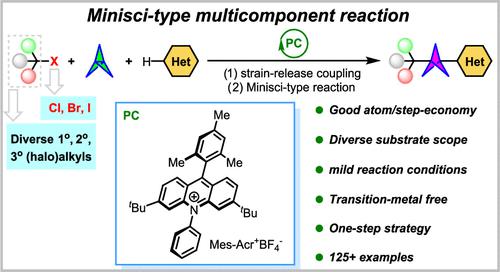
Aryl-substituted bicyclo[1.1.1]pentane (BCP-aryl) derivatives represent the most important bioisosteres of biaryl scaffolds and widely exist in numerous complex pharmaceutical molecules. The current synthetic method limitations of using only tertiary radical precursors, prefunctionalized heteroarenes, toxic transition metals, and expensive photocatalysts make it urgent to develop a more simple and practical protocol. To confront the limitations and enrich Minisci-type chemistry, herein, we disclose a photocatalytic Minisci-type multicomponent reaction for the synthesis of various (halo)alkyl BCP-aryls using [1.1.1]propellane, alkyl halides, and unfunctionalized heteroarenes as starting materials. Diverse kinds of unfunctionalized heteroarenes and alkyl radicals (primary, secondary, and tertiary carbons) derived from various alkyl chlorides, alkyl bromides, and fluoroalkyl iodides are very compatible in this transformation. The practicability of this method is additionally boosted by product derivatizations and the late-stage functionalization of pharmaceutically relevant molecules. The mechanistic studies demonstrate that a radical relay mechanism initiated by a consecutive photoinduced electron transfer (ConPET) process is in operation. We anticipate that this methodology would act as a useful tool for the synthesis of (halo)alkyl BCP-aryls as the bioisosteres of biaryl-type drug derivatives, ultimately resulting in great utility in the drug discovery program.
中文翻译:

光催化Minisci型多组分反应合成1-(卤代)烷基-3-杂芳基双环[1.1.1]戊烷
芳基取代的双环[1.1.1]戊烷(BCP-芳基)衍生物代表了联芳支架最重要的生物等排体,广泛存在于众多复杂的药物分子中。目前仅使用叔自由基前体、预官能化杂芳烃、有毒过渡金属和昂贵光催化剂的合成方法的局限性使得迫切需要开发更简单实用的方案。为了应对局限性并丰富Minisci型化学,本文中,我们公开了一种光催化Minisci型多组分反应,用于使用[1.1.1]丙烷、烷基卤和未官能化杂芳烃作为起始物合成各种(卤代)烷基BCP-芳基。材料。衍生自各种烷基氯、烷基溴和氟烷基碘的多种未官能化杂芳烃和烷基(伯碳、仲碳和叔碳)在该转化中非常相容。产品衍生化和药学相关分子的后期功能化进一步增强了该方法的实用性。机理研究表明,由连续光致电子转移(ConPET)过程引发的激进中继机制正在运行。我们预计该方法将作为合成(卤)烷基 BCP-芳基作为联芳基型药物衍生物的生物电子等排体的有用工具,最终在药物发现计划中产生巨大的实用性。
更新日期:2024-04-10
中文翻译:

光催化Minisci型多组分反应合成1-(卤代)烷基-3-杂芳基双环[1.1.1]戊烷
芳基取代的双环[1.1.1]戊烷(BCP-芳基)衍生物代表了联芳支架最重要的生物等排体,广泛存在于众多复杂的药物分子中。目前仅使用叔自由基前体、预官能化杂芳烃、有毒过渡金属和昂贵光催化剂的合成方法的局限性使得迫切需要开发更简单实用的方案。为了应对局限性并丰富Minisci型化学,本文中,我们公开了一种光催化Minisci型多组分反应,用于使用[1.1.1]丙烷、烷基卤和未官能化杂芳烃作为起始物合成各种(卤代)烷基BCP-芳基。材料。衍生自各种烷基氯、烷基溴和氟烷基碘的多种未官能化杂芳烃和烷基(伯碳、仲碳和叔碳)在该转化中非常相容。产品衍生化和药学相关分子的后期功能化进一步增强了该方法的实用性。机理研究表明,由连续光致电子转移(ConPET)过程引发的激进中继机制正在运行。我们预计该方法将作为合成(卤)烷基 BCP-芳基作为联芳基型药物衍生物的生物电子等排体的有用工具,最终在药物发现计划中产生巨大的实用性。































 京公网安备 11010802027423号
京公网安备 11010802027423号