当前位置:
X-MOL 学术
›
Nano Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The Interplay of Solvation and Polarization Effects on Ion Pairing in Nanoconfined Electrolytes
Nano Letters ( IF 9.6 ) Pub Date : 2024-04-09 , DOI: 10.1021/acs.nanolett.4c00890 Kara D Fong 1 , Barbara Sumić 1 , Niamh O'Neill 1 , Christoph Schran 2 , Clare P Grey 1 , Angelos Michaelides 1
Nano Letters ( IF 9.6 ) Pub Date : 2024-04-09 , DOI: 10.1021/acs.nanolett.4c00890 Kara D Fong 1 , Barbara Sumić 1 , Niamh O'Neill 1 , Christoph Schran 2 , Clare P Grey 1 , Angelos Michaelides 1
Affiliation
|
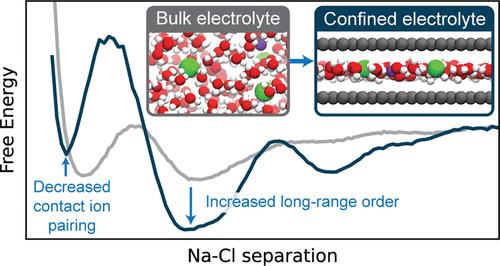
The nature of ion–ion interactions in electrolytes confined to nanoscale pores has important implications for energy storage and separation technologies. However, the physical effects dictating the structure of nanoconfined electrolytes remain debated. Here we employ machine-learning-based molecular dynamics simulations to investigate ion–ion interactions with density functional theory level accuracy in a prototypical confined electrolyte, aqueous NaCl within graphene slit pores. We find that the free energy of ion pairing in highly confined electrolytes deviates substantially from that in bulk solutions, observing a decrease in contact ion pairing but an increase in solvent-separated ion pairing. These changes arise from an interplay of ion solvation effects and graphene’s electronic structure. Notably, the behavior observed from our first-principles-level simulations is not reproduced even qualitatively with the classical force fields conventionally used to model these systems. The insight provided in this work opens new avenues for predicting and controlling the structure of nanoconfined electrolytes.
中文翻译:

溶剂化和极化效应对纳米限域电解质中离子对的相互作用
局限于纳米级孔隙的电解质中离子-离子相互作用的性质对储能和分离技术具有重要意义。然而,决定纳米限域电解质结构的物理效应仍然存在争议。在这里,我们采用基于机器学习的分子动力学模拟来研究石墨烯狭缝孔内原型受限电解质、水性 NaCl 中具有密度泛函理论级精度的离子-离子相互作用。我们发现,在高度受限的电解质中,离子对的自由能与本体溶液中的自由能有很大偏差,观察到接触离子对减少,但溶剂分离的离子对增加。这些变化是离子溶剂化效应和石墨烯电子结构相互作用的结果。值得注意的是,从我们的第一性原理级模拟中观察到的行为甚至没有用通常用于模拟这些系统的经典力场进行定性再现。这项工作中提供的见解为预测和控制纳米限域电解质的结构开辟了新的途径。
更新日期:2024-04-09
中文翻译:

溶剂化和极化效应对纳米限域电解质中离子对的相互作用
局限于纳米级孔隙的电解质中离子-离子相互作用的性质对储能和分离技术具有重要意义。然而,决定纳米限域电解质结构的物理效应仍然存在争议。在这里,我们采用基于机器学习的分子动力学模拟来研究石墨烯狭缝孔内原型受限电解质、水性 NaCl 中具有密度泛函理论级精度的离子-离子相互作用。我们发现,在高度受限的电解质中,离子对的自由能与本体溶液中的自由能有很大偏差,观察到接触离子对减少,但溶剂分离的离子对增加。这些变化是离子溶剂化效应和石墨烯电子结构相互作用的结果。值得注意的是,从我们的第一性原理级模拟中观察到的行为甚至没有用通常用于模拟这些系统的经典力场进行定性再现。这项工作中提供的见解为预测和控制纳米限域电解质的结构开辟了新的途径。

































 京公网安备 11010802027423号
京公网安备 11010802027423号