当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Multistep Reaction Mechanisms in Nonaqueous Lithium–Oxygen Batteries with Redox Mediator: A Model-Based Study
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2016-10-21 00:00:00 , DOI: 10.1021/acs.jpcc.6b07886 Daniel Grübl 1 , Benjamin Bergner 2 , Daniel Schröder 2 , Jürgen Janek 2 , Wolfgang G. Bessler 1
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2016-10-21 00:00:00 , DOI: 10.1021/acs.jpcc.6b07886 Daniel Grübl 1 , Benjamin Bergner 2 , Daniel Schröder 2 , Jürgen Janek 2 , Wolfgang G. Bessler 1
Affiliation
|
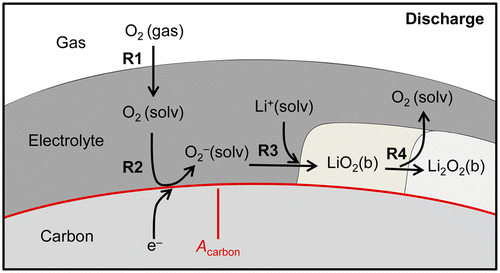
Lithium–oxygen cells with nonaqueous electrolyte show high overpotentials during charge, indicating asymmetric charge/discharge reaction mechanisms. We present a kinetic modeling and simulation study of the lithium–oxygen cell cycling behavior. The model includes a multistep reaction mechanism of the cell reaction (2Li + O2 ⇄ Li2O2) forming lithium peroxide by precipitation, coupled to a 1D porous-electrode transport model. We apply the model to study the asymmetric discharge/charge characteristics and analyze the influence of a redox mediator dissolved homogeneously in the liquid electrolyte. Model predictions are compared to experimental galvanostatic cycling data of cells without and with 2,2,6,6-tetramethylpiperidinyloxyl (TEMPO) as redox mediator. The predicted discharge behavior shows good agreement with the experimental results. A spatiotemporal analysis of species concentrations reveals inhomogeneous distributions of dissolved oxygen and reaction products within the cathode during discharge. The experimentally observed charge overpotentials as well as their reduction by using a redox mediator can be qualitatively reproduced with a partially irreversible reaction mechanism. However, the proposed models fail to reproduce the particular shape of the experimental charge curve with continuously increasing charge overpotential, which implies that part of the reaction mechanism is still open for investigation in future work.
中文翻译:

非水锂氧电池中氧化还原介体的多步反应机理:基于模型的研究
带有非水电解质的锂氧电池在充电过程中显示出很高的过电势,表明存在不对称的充电/放电反应机理。我们提出了锂氧电池循环行为的动力学建模和仿真研究。该模型包括细胞反应的多步反应机理(2LI + O 2 ⇄栗2 ö 2)通过沉淀形成过氧化锂,并与一维多孔电极传输模型耦合。我们将该模型用于研究非对称放电/充电特性,并分析了均匀溶解在液体电解质中的氧化还原介体的影响。将模型预测与没有和有2,2,6,6-四甲基哌啶基氧基(TEMPO)作为氧化还原介体的细胞的实验恒电流循环数据进行比较。预测的放电行为与实验结果显示出良好的一致性。物种浓度的时空分析表明,放电期间阴极内溶解氧和反应产物的分布不均匀。实验观察到的电荷超电势及其通过使用氧化还原介体的还原可通过部分不可逆的反应机制定性地再现。然而,所提出的模型无法随着电荷超电势的不断增加而重现实验电荷曲线的特殊形状,这意味着该反应机理的一部分仍在开放中,以供将来的研究之用。
更新日期:2016-10-21
中文翻译:

非水锂氧电池中氧化还原介体的多步反应机理:基于模型的研究
带有非水电解质的锂氧电池在充电过程中显示出很高的过电势,表明存在不对称的充电/放电反应机理。我们提出了锂氧电池循环行为的动力学建模和仿真研究。该模型包括细胞反应的多步反应机理(2LI + O 2 ⇄栗2 ö 2)通过沉淀形成过氧化锂,并与一维多孔电极传输模型耦合。我们将该模型用于研究非对称放电/充电特性,并分析了均匀溶解在液体电解质中的氧化还原介体的影响。将模型预测与没有和有2,2,6,6-四甲基哌啶基氧基(TEMPO)作为氧化还原介体的细胞的实验恒电流循环数据进行比较。预测的放电行为与实验结果显示出良好的一致性。物种浓度的时空分析表明,放电期间阴极内溶解氧和反应产物的分布不均匀。实验观察到的电荷超电势及其通过使用氧化还原介体的还原可通过部分不可逆的反应机制定性地再现。然而,所提出的模型无法随着电荷超电势的不断增加而重现实验电荷曲线的特殊形状,这意味着该反应机理的一部分仍在开放中,以供将来的研究之用。






























 京公网安备 11010802027423号
京公网安备 11010802027423号