当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enzymatic Fluoroethylation by a Fluoroethyl Selenium Analogue of S-Adenosylmethionine
ACS Catalysis ( IF 11.3 ) Pub Date : 2024-04-09 , DOI: 10.1021/acscatal.4c01112
Nanhai Yu 1 , Huimin Zhao 1 , Wenrui Wang 1 , Min Dong 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2024-04-09 , DOI: 10.1021/acscatal.4c01112
Nanhai Yu 1 , Huimin Zhao 1 , Wenrui Wang 1 , Min Dong 1
Affiliation
|
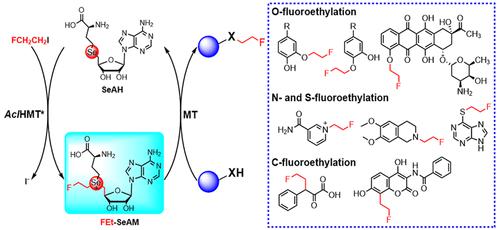
Fluorine is a unique element with important roles in medicinal chemistry, agrochemistry, and materials chemistry. The fluoroethyl group is an important fluoroalkyl functional unit that is widely used in clinical drugs, 19F probes and 18F PET diagnostic drugs. Chemo- and regioselective fluoroethylation is difficult in chemical synthesis. To date, no enzymatic reaction for selective fluoroethylation has been reported. Based on the widespread natural methyl donor S-adenosine-l-methionine (SAM), we designed and synthesized a fluoroethyl SAM analogue (FEt-SAM). A stability study revealed that FEt-SAM was very labile under physiological conditions and gave the fluorine-elimination product vinyl-SAM. We circumvented this problem by replacing the S in FEt-SAM with Se to give fluoroethyl Se-adenosyl-l-selenomethionine (FEt-SeAM). By using halide methyltransferase (HMT) and its mutant for the in situ production of FEt-SeAM, we created cascade reactions of the HMT mutant with methyltransferases and fluoroethylated several O-, N-, S-, and C-nucleophiles. For methyltransferases that did not recognize FEt-SeAM well, such as DnrK and NovO, simple mutagenesis of the conserved hydrophobic residues (Leu and Ile) in the SAM binding pocket to smaller amino acids significantly increased the activities. Therefore, we have provided a useful tool for the late-stage fluoroethylation of natural products and drugs. This method could also be used to enzymatically prepare probes for 19F NMR and 18F PET tests.
中文翻译:

S-腺苷甲硫氨酸的氟乙基硒类似物的酶促氟乙基化
氟是一种独特的元素,在药物化学、农业化学和材料化学中发挥着重要作用。氟乙基是重要的氟烷基功能单元,广泛应用于临床药物、19 F探针和18 F PET诊断药物中。化学和区域选择性氟乙基化在化学合成中很困难。迄今为止,尚未报道选择性氟乙基化的酶促反应。基于广泛存在的天然甲基供体S-腺苷-l-蛋氨酸(SAM),我们设计并合成了氟乙基SAM类似物(FEt-SAM)。稳定性研究表明,FEt-SAM 在生理条件下非常不稳定,并产生了除氟产物乙烯基-SAM。我们通过用 Se 替换 FEt-SAM 中的 S 来解决这个问题,得到氟乙基 Se-腺苷-l-硒代蛋氨酸 (FEt-SeAM)。通过使用卤化物甲基转移酶 (HMT) 及其突变体原位生产 FEt-SeAM,我们创建了 HMT 突变体与甲基转移酶的级联反应,并对几种 O-、N-、S- 和 C-亲核试剂进行氟乙基化。对于不能很好地识别 FEt-SeAM 的甲基转移酶,例如 DnrK 和 NovO,将 SAM 结合袋中的保守疏水残基(Leu 和 Ile)简单诱变为较小的氨基酸可显着提高活性。因此,我们为天然产物和药物的后期氟乙基化提供了有用的工具。该方法还可用于酶法制备用于19 F NMR 和18 F PET 测试的探针。
更新日期:2024-04-09
中文翻译:

S-腺苷甲硫氨酸的氟乙基硒类似物的酶促氟乙基化
氟是一种独特的元素,在药物化学、农业化学和材料化学中发挥着重要作用。氟乙基是重要的氟烷基功能单元,广泛应用于临床药物、19 F探针和18 F PET诊断药物中。化学和区域选择性氟乙基化在化学合成中很困难。迄今为止,尚未报道选择性氟乙基化的酶促反应。基于广泛存在的天然甲基供体S-腺苷-l-蛋氨酸(SAM),我们设计并合成了氟乙基SAM类似物(FEt-SAM)。稳定性研究表明,FEt-SAM 在生理条件下非常不稳定,并产生了除氟产物乙烯基-SAM。我们通过用 Se 替换 FEt-SAM 中的 S 来解决这个问题,得到氟乙基 Se-腺苷-l-硒代蛋氨酸 (FEt-SeAM)。通过使用卤化物甲基转移酶 (HMT) 及其突变体原位生产 FEt-SeAM,我们创建了 HMT 突变体与甲基转移酶的级联反应,并对几种 O-、N-、S- 和 C-亲核试剂进行氟乙基化。对于不能很好地识别 FEt-SeAM 的甲基转移酶,例如 DnrK 和 NovO,将 SAM 结合袋中的保守疏水残基(Leu 和 Ile)简单诱变为较小的氨基酸可显着提高活性。因此,我们为天然产物和药物的后期氟乙基化提供了有用的工具。该方法还可用于酶法制备用于19 F NMR 和18 F PET 测试的探针。

































 京公网安备 11010802027423号
京公网安备 11010802027423号