当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Development of a Continuous Process for the Large-Scale Asymmetric Manufacture of (R)-3-Methoxy-2-(4-methylpiperazin-1-yl)propanoic Acid
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2024-04-08 , DOI: 10.1021/acs.oprd.3c00409 Carl J. Mallia 1 , Peter R. Moore 1 , Simon Hardy 1 , Christopher D. Parsons 1 , Paul A. J. Cronin 1 , Andrew Ikin 1 , Carl-Johan Aurell 2 , Kuangchu Dai 3 , Baoquan Sun 3
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2024-04-08 , DOI: 10.1021/acs.oprd.3c00409 Carl J. Mallia 1 , Peter R. Moore 1 , Simon Hardy 1 , Christopher D. Parsons 1 , Paul A. J. Cronin 1 , Andrew Ikin 1 , Carl-Johan Aurell 2 , Kuangchu Dai 3 , Baoquan Sun 3
Affiliation
|
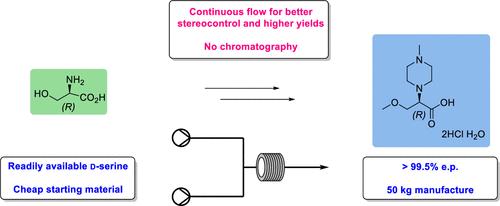
A large-scale enantioselective manufacturing route to an unusual piperazine-substituted amino acid is described. Previous synthetic routes to this amino acid relied on the resolution of racemic mixtures using l-tartaric acid that was demonstrated on a 6 kg scale, but this resulted in a reduced overall yield and efficiency. The new enantioselective route to this amino acid uses the SN2 displacement of a chiral triflate with N-methylpiperazine that proceeds with very high levels of stereocontrol. The key chiral triflate is prepared in five synthetic steps in 38% overall yield and >99% enantiomeric purity (e.p.), starting from cheap and readily available d-serine. Subsequent reaction with N-methylpiperazine was initially demonstrated in batch, providing the benzyl-protected amino acid in 83% e.p. on a 3 kg scale. This transformation was further improved by the application of continuous manufacture to provide the benzyl-protected ester in >99% e.p. on an 80 kg scale. Simple deprotection of the benzyl ester group by hydrogenolysis, followed by isolation of the amino acid as the corresponding dihydrochloride salt, provided a scalable and efficient synthesis of (R)-3-methoxy-2-(4-methylpiperazin-1-yl)propanoic acid in good overall yield (33%) and very high optical purity (>99.5% e.p.).
中文翻译:

开发大规模不对称生产 (R)-3-甲氧基-2-(4-甲基哌嗪-1-基)丙酸的连续工艺
描述了一种罕见的哌嗪取代氨基酸的大规模对映选择性生产路线。以前这种氨基酸的合成路线依赖于使用L-酒石酸拆分外消旋混合物,这在 6 kg 规模上得到了证实,但这导致了总体产量和效率的降低。该氨基酸的新对映选择性路线使用手性三氟甲磺酸酯与N-甲基哌嗪的 S N 2 置换,从而进行非常高水平的立体控制。关键的手性三氟甲磺酸酯是从廉价且容易获得的d-丝氨酸开始,通过五个合成步骤制备的,总收率 38%,对映体纯度 (ep) > 99% 。随后与N-甲基哌嗪的反应最初在批量中进行了演示,以 3 kg 规模提供了 83% ep 的苄基保护氨基酸。通过连续生产的应用进一步改进了这种转化,以在 80 公斤规模上提供 >99% ep 的苄基保护酯。通过氢解对苄酯基团进行简单脱保护,然后将氨基酸分离为相应的二盐酸盐,提供了 ( R )-3-甲氧基-2-(4-甲基哌嗪-1-基)丙酸的可扩展且高效的合成酸具有良好的总收率 (33%) 和非常高的光学纯度 (>99.5% ep)。
更新日期:2024-04-08
中文翻译:

开发大规模不对称生产 (R)-3-甲氧基-2-(4-甲基哌嗪-1-基)丙酸的连续工艺
描述了一种罕见的哌嗪取代氨基酸的大规模对映选择性生产路线。以前这种氨基酸的合成路线依赖于使用L-酒石酸拆分外消旋混合物,这在 6 kg 规模上得到了证实,但这导致了总体产量和效率的降低。该氨基酸的新对映选择性路线使用手性三氟甲磺酸酯与N-甲基哌嗪的 S N 2 置换,从而进行非常高水平的立体控制。关键的手性三氟甲磺酸酯是从廉价且容易获得的d-丝氨酸开始,通过五个合成步骤制备的,总收率 38%,对映体纯度 (ep) > 99% 。随后与N-甲基哌嗪的反应最初在批量中进行了演示,以 3 kg 规模提供了 83% ep 的苄基保护氨基酸。通过连续生产的应用进一步改进了这种转化,以在 80 公斤规模上提供 >99% ep 的苄基保护酯。通过氢解对苄酯基团进行简单脱保护,然后将氨基酸分离为相应的二盐酸盐,提供了 ( R )-3-甲氧基-2-(4-甲基哌嗪-1-基)丙酸的可扩展且高效的合成酸具有良好的总收率 (33%) 和非常高的光学纯度 (>99.5% ep)。































 京公网安备 11010802027423号
京公网安备 11010802027423号