当前位置:
X-MOL 学术
›
ACS Med. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Discovery of Disubstituted Carboranes as Inhibitors of Heat Shock Protein 90–Heat Shock Factor 1 Interaction
ACS Medicinal Chemistry Letters ( IF 3.5 ) Pub Date : 2024-04-08 , DOI: 10.1021/acsmedchemlett.4c00022 Yujie Shao 1 , Kazuki Miura 1, 2 , Yasunobu Asawa 1 , Taiki Morita 1, 2 , Guangzhe Li 3 , Hiroyuki Nakamura 1, 2
ACS Medicinal Chemistry Letters ( IF 3.5 ) Pub Date : 2024-04-08 , DOI: 10.1021/acsmedchemlett.4c00022 Yujie Shao 1 , Kazuki Miura 1, 2 , Yasunobu Asawa 1 , Taiki Morita 1, 2 , Guangzhe Li 3 , Hiroyuki Nakamura 1, 2
Affiliation
|
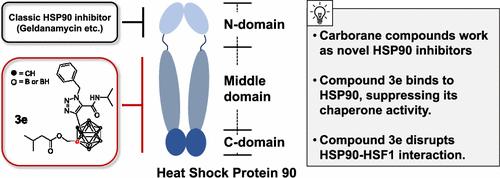
Efficient synthesis of disubstituted para- and ortho-carboranes (2 and 3, respectively) was achieved. Among the compounds synthesized, 3e showed potent suppression of hypoxia-inducible factor 1 (HIF-1) transcriptional activity under hypoxia by a cell-based reporter gene assay. Detailed mechanism-of-action studies revealed that 3e reduced the stability of heat shock protein (HSP) 90 client proteins such as CDK4, AKT, and cyclin D1 by inhibiting HSP90 chaperone activity but did not induce a heat shock response (HSR), which may cause drug resistance. Furthermore, 3e inhibited the interaction between HSP90 and heat shock factor 1 (HSF1), resulting in reducing HSF1 protein stability and thereby suppressing the transcription of heat shock proteins.
中文翻译:

发现二取代碳硼烷作为热休克蛋白 90-热休克因子 1 相互作用的抑制剂
实现了双取代的对位碳硼烷和邻位碳硼烷(分别为2和3 )的高效合成。在合成的化合物中,通过基于细胞的报告基因检测, 3e在缺氧条件下显示出对缺氧诱导因子 1 (HIF-1) 转录活性的有效抑制。详细的作用机制研究表明, 3e通过抑制 HSP90 伴侣活性来降低热休克蛋白 (HSP) 90 客户蛋白(例如 CDK4、AKT 和细胞周期蛋白 D1)的稳定性,但不会诱导热休克反应 (HSR),这可能会引起耐药性。此外, 3e抑制 HSP90 和热休克因子 1 (HSF1) 之间的相互作用,导致 HSF1 蛋白稳定性降低,从而抑制热休克蛋白的转录。
更新日期:2024-04-08
中文翻译:

发现二取代碳硼烷作为热休克蛋白 90-热休克因子 1 相互作用的抑制剂
实现了双取代的对位碳硼烷和邻位碳硼烷(分别为2和3 )的高效合成。在合成的化合物中,通过基于细胞的报告基因检测, 3e在缺氧条件下显示出对缺氧诱导因子 1 (HIF-1) 转录活性的有效抑制。详细的作用机制研究表明, 3e通过抑制 HSP90 伴侣活性来降低热休克蛋白 (HSP) 90 客户蛋白(例如 CDK4、AKT 和细胞周期蛋白 D1)的稳定性,但不会诱导热休克反应 (HSR),这可能会引起耐药性。此外, 3e抑制 HSP90 和热休克因子 1 (HSF1) 之间的相互作用,导致 HSF1 蛋白稳定性降低,从而抑制热休克蛋白的转录。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号