当前位置:
X-MOL 学术
›
Chem. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
La3Li3W2O12: Ionic Diffusion in a Perovskite with Lithium on both A- and B-Sites
Chemistry of Materials ( IF 7.2 ) Pub Date : 2016-10-21 00:00:00 , DOI: 10.1021/acs.chemmater.6b03220
Alma B. Santibáñez-Mendieta 1 , Christophe Didier 1 , Kenneth K. Inglis 1 , Alex J. Corkett 1 , Michael J. Pitcher 1 , Marco Zanella 1 , J. Felix Shin 1 , Luke M. Daniels 1 , Aydar Rakhmatullin 2 , Ming Li 3 , Matthew S. Dyer 1 , John B. Claridge 1 , Frédéric Blanc 1, 4 , Matthew J. Rosseinsky 1
Chemistry of Materials ( IF 7.2 ) Pub Date : 2016-10-21 00:00:00 , DOI: 10.1021/acs.chemmater.6b03220
Alma B. Santibáñez-Mendieta 1 , Christophe Didier 1 , Kenneth K. Inglis 1 , Alex J. Corkett 1 , Michael J. Pitcher 1 , Marco Zanella 1 , J. Felix Shin 1 , Luke M. Daniels 1 , Aydar Rakhmatullin 2 , Ming Li 3 , Matthew S. Dyer 1 , John B. Claridge 1 , Frédéric Blanc 1, 4 , Matthew J. Rosseinsky 1
Affiliation
|
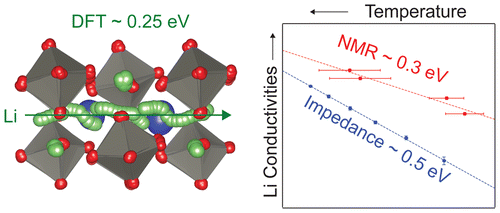
The structure and Li+ ion dynamics of a new class of ABO3 perovskite with Li on both the A- and B-sites are described. La3Li3W2O12 is synthesized by solid state reaction at 900 °C and shown by powder X-ray diffraction to adopt the structure of a monoclinic double perovskite (A2)BB′O6, (La1.5Li0.5)WLiO6, with rock salt order of W6+ and Li+ on the B-site. High resolution powder neutron diffraction locates A-site Li in a distorted tetrahedron displaced from the conventional perovskite A-site, which differs considerably from the sites occupied by Li in the well studied La2/3–xLi3xTiO3 family. This is confirmed by the observation of a lower coordinated Li+ ion in the 6Li magic angle spinning nuclear magnetic resonance (NMR) spectra, in addition to the B-site LiO6, and supported computationally by density functional theory (DFT), which also suggests local order of A-site La3+ and Li+. DFT shows that the vacancies necessary for transport can arise from Frenkel or La excess defects, with an energetic cost of ∼0.4 eV/vacancy in both cases. Ab initio molecular dynamics establishes that the Li+ ion dynamics occur by a pathway involving a series of multiple localized Li hops between two neighboring A-sites with an overall energy barrier of ∼0.25 eV, with additional possible pathways involving Li exchange between the A- and B-sites. A similar activation energy for Li+ ion mobility (∼0.3 eV) was obtained from variable temperature 6Li and 7Li line narrowing and relaxometry NMR experiments, suggesting that the barrier to Li hopping between sites in La3Li3W2O12 is comparable to the best oxide Li+ ion conductors. AC impedance-derived conductivities confirm that Li+ ions are mobile but that the long-range Li+ diffusion has a higher barrier (∼0.5 eV) which may be associated with blocking of transport by A-site La3+ ions.
中文翻译:

La 3 Li 3 W 2 O 12:钙钛矿中锂在A和B站点上的离子扩散
描述了新型ABO 3钙钛矿与Li在A位和B位上的结构和Li +离子动力学。La 3 Li 3 W 2 O 12在900°C下通过固相反应合成,并通过粉末X射线衍射显示采用单斜晶钙钛矿(A 2)BB'O 6的结构,(La 1.5 Li 0.5) WLiO 6,岩盐顺序为W 6+和Li +在B网站上。高分辨率粉末中子衍射将A位置的Li定位在与常规钙钛矿A位置不同的变形四面体中,这与经过充分研究的La 2 / 3– x Li 3 x TiO 3族中Li所占据的位置有很大不同。除B位LiO 6外,还通过在6 Li魔角旋转核磁共振(NMR)光谱中观察到较低的配位Li +离子,并得到密度泛函理论(DFT)的计算支持,证实了这一点。也暗示了A站点La 3+和Li +的局部顺序。DFT显示,运输所需的空位可能由Frenkel或La过量缺陷引起,两种情况下的能源成本约为0.4 eV /空位。从头算分子动力学确定,Li +离子动力学是通过一个路径发生的,该路径涉及两个相邻A位之间的一系列多个局部Li跃点,总能垒约为0.25 eV,其他可能的路径涉及A-之间的Li交换和B网站。从可变温度的6 Li和7 Li谱线变窄和弛豫法NMR实验中获得了与Li +离子迁移率相似的活化能(〜0.3 eV),这表明在La 3 Li 3中位点之间跳跃跳跃的障碍W 2 O 12可与最佳氧化物Li +离子导体媲美。交流阻抗衍生的电导率证实了Li +离子是可移动的,但长距离Li +扩散具有较高的势垒(〜0.5 eV),这可能与A位La 3+离子的运输受阻有关。
更新日期:2016-10-21
中文翻译:

La 3 Li 3 W 2 O 12:钙钛矿中锂在A和B站点上的离子扩散
描述了新型ABO 3钙钛矿与Li在A位和B位上的结构和Li +离子动力学。La 3 Li 3 W 2 O 12在900°C下通过固相反应合成,并通过粉末X射线衍射显示采用单斜晶钙钛矿(A 2)BB'O 6的结构,(La 1.5 Li 0.5) WLiO 6,岩盐顺序为W 6+和Li +在B网站上。高分辨率粉末中子衍射将A位置的Li定位在与常规钙钛矿A位置不同的变形四面体中,这与经过充分研究的La 2 / 3– x Li 3 x TiO 3族中Li所占据的位置有很大不同。除B位LiO 6外,还通过在6 Li魔角旋转核磁共振(NMR)光谱中观察到较低的配位Li +离子,并得到密度泛函理论(DFT)的计算支持,证实了这一点。也暗示了A站点La 3+和Li +的局部顺序。DFT显示,运输所需的空位可能由Frenkel或La过量缺陷引起,两种情况下的能源成本约为0.4 eV /空位。从头算分子动力学确定,Li +离子动力学是通过一个路径发生的,该路径涉及两个相邻A位之间的一系列多个局部Li跃点,总能垒约为0.25 eV,其他可能的路径涉及A-之间的Li交换和B网站。从可变温度的6 Li和7 Li谱线变窄和弛豫法NMR实验中获得了与Li +离子迁移率相似的活化能(〜0.3 eV),这表明在La 3 Li 3中位点之间跳跃跳跃的障碍W 2 O 12可与最佳氧化物Li +离子导体媲美。交流阻抗衍生的电导率证实了Li +离子是可移动的,但长距离Li +扩散具有较高的势垒(〜0.5 eV),这可能与A位La 3+离子的运输受阻有关。





































 京公网安备 11010802027423号
京公网安备 11010802027423号