当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Recognizing Functional Groups of MES/APG Mixed Surfactants for Enhanced Solubilization toward Benzo[a]pyrene
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2024-04-04 , DOI: 10.1021/acs.est.3c10633 Liyuan Wu 1, 2, 3 , Yaxin Liu 1, 2, 3 , Xin Wang 4 , Mengrui Li 1, 2, 3 , Jingya Li 1, 2, 3 , Xiaoran Zhang 1, 2, 3 , Dawen Gao 1, 2, 3 , Haiyan Li 1, 2, 3
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2024-04-04 , DOI: 10.1021/acs.est.3c10633 Liyuan Wu 1, 2, 3 , Yaxin Liu 1, 2, 3 , Xin Wang 4 , Mengrui Li 1, 2, 3 , Jingya Li 1, 2, 3 , Xiaoran Zhang 1, 2, 3 , Dawen Gao 1, 2, 3 , Haiyan Li 1, 2, 3
Affiliation
|
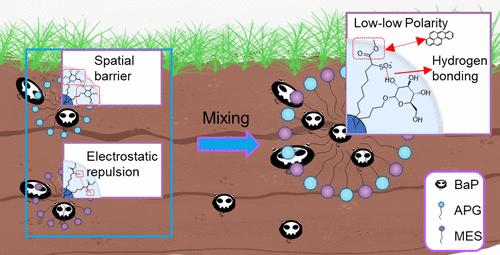
Benzo[a]pyrene is difficult to remove from soil due to its high octanol/water partition coefficient. The use of mixed surfactants can increase solubility but with the risk of secondary soil contamination, and the compounding mechanism is still unclear. This study introduced a new approach using environmentally friendly fatty acid methyl ester sulfonate (MES) and alkyl polyglucoside (APG) to solubilize benzo[a]pyrene. The best result was obtained when the ratio of MES/APG was 7:1 under 6 g/L total concentration, with an apparent solubility (Sw) of 8.58 mg/L and a molar solubilization ratio (MSR) of 1.31 for benzo[a]pyrene, which is comparable to that of Tween 80 (MSR, 0.95). The mechanism indicates that the hydroxyl groups (−OH) in APG form “O–H···OSO2–” hydrogen bonding with the sulfonic acid group (−SO3–) of MES, which reduces the electrostatic repulsion between MES molecules, thus facilitating the formation of large and stable micelles. Moreover, the strong solubilizing effect on benzo[a]pyrene should be ascribed to the low polarity of ester groups (−COOCH3) in MES. Functional groups capable of forming hydrogen bonds and having low polarity are responsible for the enhanced solubilization of benzo[a]pyrene. This understanding helps choose suitable surfactants for the remediation of PAH-contaminated soils.
中文翻译:

识别 MES/APG 混合表面活性剂的官能团以增强对苯并[a]芘的溶解
由于苯并[ a ]芘的辛醇/水分配系数较高,因此很难从土壤中去除。使用混合表面活性剂可以提高溶解度,但存在二次土壤污染的风险,且复配机理尚不清楚。本研究介绍了一种使用环保的脂肪酸甲酯磺酸盐(MES)和烷基多糖苷(APG)溶解苯并[ a ]芘的新方法。当MES/APG比为7:1时,总浓度为6 g/L,苯并[的表观溶解度( S w )为8.58 mg/L,摩尔增溶比(MSR)为1.31]。 a ]芘,与 Tween 80 相当(MSR,0.95)。机理表明APG中的羟基(−OH)与MES的磺酸基(−SO 3 – )形成“O–H···OSO 2 - ”氢键,降低了MES分子间的静电斥力,从而促进大而稳定的胶束的形成。此外,对苯并[ a ]芘的强增溶作用应归因于MES中酯基(-COOCH 3 )的低极性。能够形成氢键并具有低极性的官能团负责增强苯并[ a ]芘的溶解。这种理解有助于选择合适的表面活性剂来修复多环芳烃污染的土壤。
更新日期:2024-04-04
中文翻译:

识别 MES/APG 混合表面活性剂的官能团以增强对苯并[a]芘的溶解
由于苯并[ a ]芘的辛醇/水分配系数较高,因此很难从土壤中去除。使用混合表面活性剂可以提高溶解度,但存在二次土壤污染的风险,且复配机理尚不清楚。本研究介绍了一种使用环保的脂肪酸甲酯磺酸盐(MES)和烷基多糖苷(APG)溶解苯并[ a ]芘的新方法。当MES/APG比为7:1时,总浓度为6 g/L,苯并[的表观溶解度( S w )为8.58 mg/L,摩尔增溶比(MSR)为1.31]。 a ]芘,与 Tween 80 相当(MSR,0.95)。机理表明APG中的羟基(−OH)与MES的磺酸基(−SO 3 – )形成“O–H···OSO 2 - ”氢键,降低了MES分子间的静电斥力,从而促进大而稳定的胶束的形成。此外,对苯并[ a ]芘的强增溶作用应归因于MES中酯基(-COOCH 3 )的低极性。能够形成氢键并具有低极性的官能团负责增强苯并[ a ]芘的溶解。这种理解有助于选择合适的表面活性剂来修复多环芳烃污染的土壤。































 京公网安备 11010802027423号
京公网安备 11010802027423号