当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
[CuO]+ and [CuOH]2+ Complexes: Intermediates in Oxidation Catalysis?
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2015-06-15 00:00:00 , DOI: 10.1021/acs.accounts.5b00169 Nicole Gagnon 1 , William B. Tolman 1
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2015-06-15 00:00:00 , DOI: 10.1021/acs.accounts.5b00169 Nicole Gagnon 1 , William B. Tolman 1
Affiliation
|
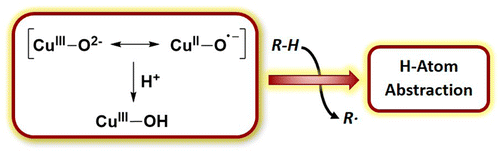
Characterization of monocopper intermediates in enzymes and other catalysts that attack strong C–H bonds is important for unraveling oxidation catalysis mechanisms and, ultimately, designing new, more efficient catalytic systems. Because initially formed 1:1 Cu/O2 adducts resulting from reactions of Cu(I) sites with O2 react relatively sluggishly with substrates with strong C–H bonds, it has been suggested that reductive O–O bond scission might occur instead to yield more reactive [CuO]+ or protonated [CuOH]2+ cores. Experimental and theoretical studies of [CuO]+ species in the gas phase have provided key insights into the possible reactivity of these species, but detailed information is lacking for discrete complexes with the [CuO]+ or [CuOH]2+ core in solution or the solid state. We describe herein our recent efforts to address this issue through several disparate approaches. In one strategy based on precedent from studies of enzymes and synthetic compounds with iron-α-ketocarboxylate motifs, reactions of O2 with Cu(I)-α-ketocarboxylate complexes were explored, with the aim of identifying reaction pathways that would implicate the intermediacy of a [CuO]+ species. A second approach focused on the reaction of N-oxides with Cu(I) complexes, with the goal being to elicit O–N bond heterolysis to yield [CuO]+ complexes. For both strategies, the course of the reactions depended on the nature of the supporting bidentate N-donor ligand, and indirect evidence in support of the sought-after [CuO]+ intermediates was obtained in some instances.
中文翻译:

[CuO] +和[CuOH] 2+配合物:氧化催化中的中间体?
酶和其他攻击强C–H键的催化剂中的单铜中间体的表征对于揭示氧化催化机理以及最终设计新的更有效的催化体系非常重要。由于最初形成的1:1 Cu / O 2加合物是由Cu(I)位与O 2反应生成的,因此与具有强C-H键的底物反应较慢,因此建议还原O-O键可能会断裂。产生更多的反应性[CuO] +或质子化的[CuOH] 2+核。[CuO] +的实验和理论研究气相中的某些物种提供了对这些物种可能的反应性的关键见解,但缺少与[CuO] +或[CuOH] 2+核呈溶液或固态的离散复合物的详细信息。我们在这里描述了我们最近通过几种不同的方法来解决这个问题的努力。在一项基于对酶和具有铁-α-酮基羧酸酯基序的合成化合物进行研究的先例的策略中,探索了O 2与Cu(I)-α-酮基羧酸酯络合物的反应,目的是确定暗示该中间体的反应途径。 [CuO] +物种。第二种方法集中于N-氧化物与Cu(I)配合物的反应,目的是引起O–N键杂化以产生[CuO] +配合物。对于这两种策略,反应过程都取决于支持的双齿N-供体配体的性质,在某些情况下获得了间接支持[CuO] +中间体的证据。
更新日期:2015-06-15
中文翻译:

[CuO] +和[CuOH] 2+配合物:氧化催化中的中间体?
酶和其他攻击强C–H键的催化剂中的单铜中间体的表征对于揭示氧化催化机理以及最终设计新的更有效的催化体系非常重要。由于最初形成的1:1 Cu / O 2加合物是由Cu(I)位与O 2反应生成的,因此与具有强C-H键的底物反应较慢,因此建议还原O-O键可能会断裂。产生更多的反应性[CuO] +或质子化的[CuOH] 2+核。[CuO] +的实验和理论研究气相中的某些物种提供了对这些物种可能的反应性的关键见解,但缺少与[CuO] +或[CuOH] 2+核呈溶液或固态的离散复合物的详细信息。我们在这里描述了我们最近通过几种不同的方法来解决这个问题的努力。在一项基于对酶和具有铁-α-酮基羧酸酯基序的合成化合物进行研究的先例的策略中,探索了O 2与Cu(I)-α-酮基羧酸酯络合物的反应,目的是确定暗示该中间体的反应途径。 [CuO] +物种。第二种方法集中于N-氧化物与Cu(I)配合物的反应,目的是引起O–N键杂化以产生[CuO] +配合物。对于这两种策略,反应过程都取决于支持的双齿N-供体配体的性质,在某些情况下获得了间接支持[CuO] +中间体的证据。

































 京公网安备 11010802027423号
京公网安备 11010802027423号