当前位置:
X-MOL 学术
›
Adv. Funct. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cascade Carrier-Free Nanoparticles Forming In Situ Nanovaccines for Synergistic Photothermal-Immunotherapy of Cancer
Advanced Functional Materials ( IF 18.5 ) Pub Date : 2024-04-05 , DOI: 10.1002/adfm.202401489 Chenlu Huang 1 , Hanyong Wang 1 , Xinyu Yang 1 , Qingyu Yu 1 , Hai Wang 1 , Linhua Zhang 1 , Yanli Zhao 2 , Dunwan Zhu 1
Advanced Functional Materials ( IF 18.5 ) Pub Date : 2024-04-05 , DOI: 10.1002/adfm.202401489 Chenlu Huang 1 , Hanyong Wang 1 , Xinyu Yang 1 , Qingyu Yu 1 , Hai Wang 1 , Linhua Zhang 1 , Yanli Zhao 2 , Dunwan Zhu 1
Affiliation
|
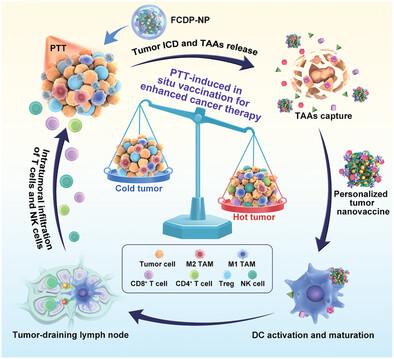
Rapid advances in nanotechnology have made it possible to combine photothermal therapy (PTT) with immunotherapy, enabling to activate an in situ vaccine effect. However, this effect is severely impeded by low antigen presentation level and highly suppressive tumor immune microenvironment (immune “cold” tumors). To overcome the obstacles, multifunctional carrier-free nanoparticles (FCDP-NPs) assembled from Fe2+, toll-like receptor 9 agonist (CpG), cationic lipid (DOTAP) and photothermal agent polydopamine are developed. After intratumoral injection, FCDP-NPs carrying positive charge are exposed under laser irradiation, which can capture tumor-associated antigens (TAAs) generated upon post-PTT to form the nanovaccines (FCD-NPs@TAAs). The nanovaccines further promote cross-presentation of TAAs, stimulate adaptive immune responses, and shape immune “hot” tumors. As a result, in situ nanovaccines highly improve survival rates and elicit a durable immune memory that remarkedly prevents tumor metastasis, illustrating a useful platform for PTT synergized with immunotherapy.
中文翻译:

级联无载体纳米粒子原位形成纳米疫苗用于癌症的协同光热免疫治疗
纳米技术的快速发展使得光热疗法(PTT)与免疫疗法相结合成为可能,从而能够激活原位疫苗效应。然而,这种效应受到低抗原呈递水平和高度抑制的肿瘤免疫微环境(免疫“冷”肿瘤)的严重阻碍。为了克服这些障碍,开发了由Fe 2+ 、Toll样受体9激动剂(CpG)、阳离子脂质(DOTAP)和光热剂聚多巴胺组装而成的多功能无载体纳米粒子(FCDP-NPs)。瘤内注射后,携带正电荷的FCDP-NPs在激光照射下暴露,可以捕获PTT后产生的肿瘤相关抗原(TAA),形成纳米疫苗(FCD-NPs@TAAs)。纳米疫苗进一步促进 TAA 的交叉呈递,刺激适应性免疫反应,并形成免疫“热”肿瘤。因此,原位纳米疫苗极大地提高了存活率,并引发持久的免疫记忆,显着防止肿瘤转移,这说明了 PTT 与免疫疗法协同作用的有用平台。
更新日期:2024-04-05
中文翻译:

级联无载体纳米粒子原位形成纳米疫苗用于癌症的协同光热免疫治疗
纳米技术的快速发展使得光热疗法(PTT)与免疫疗法相结合成为可能,从而能够激活原位疫苗效应。然而,这种效应受到低抗原呈递水平和高度抑制的肿瘤免疫微环境(免疫“冷”肿瘤)的严重阻碍。为了克服这些障碍,开发了由Fe 2+ 、Toll样受体9激动剂(CpG)、阳离子脂质(DOTAP)和光热剂聚多巴胺组装而成的多功能无载体纳米粒子(FCDP-NPs)。瘤内注射后,携带正电荷的FCDP-NPs在激光照射下暴露,可以捕获PTT后产生的肿瘤相关抗原(TAA),形成纳米疫苗(FCD-NPs@TAAs)。纳米疫苗进一步促进 TAA 的交叉呈递,刺激适应性免疫反应,并形成免疫“热”肿瘤。因此,原位纳米疫苗极大地提高了存活率,并引发持久的免疫记忆,显着防止肿瘤转移,这说明了 PTT 与免疫疗法协同作用的有用平台。































 京公网安备 11010802027423号
京公网安备 11010802027423号