Theoretical Chemistry Accounts ( IF 1.6 ) Pub Date : 2024-04-04 , DOI: 10.1007/s00214-024-03110-3 Olena O. Pylypenko , Liudmyla K. Sviatenko , Kostyantin P. Shabelnyk , Sergiy I. Kovalenko , Sergiy I. Okovytyy
|
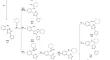
The derivatives of 1,2,4- triazole have attracted great attention among medicinal chemists due to their wide range of biological activity, good pharmacodynamic and pharmacokinetic profiles, and low toxicity, that necessitates the development of various synthesis methods and a comprehensive study of their reaction mechanisms. A detailed investigation of possible pathways for formation of new spiro-condensed [1,2,4]triazolo[1,5-c]quinazolines, that combine two structural domains with different biological properties, was performed by computational study at the SMD/B3lyp/6-31+G(d) theory level. The mechanism of interaction between [2-(3-hetaryl-1,2,4-triazol-5-yl)phenyl]amine and cyclohexanone in methanol involves three main processes: formation of carbinolamine by addition of an amine to double bond C=O, elimination of a water molecule, and intramolecular cyclization leading to formation of spiro compounds. Results show increase in reactivity of reactants during acid-catalyzed reaction compared to uncatalyzed one. The nature of the heterocyclic substituent on the triazole ring has little effect on the reaction energy, while the mechanism is unchanged.
中文翻译:

[2-(3-杂芳基-1,2,4-三唑-5-基)苯基]胺与酮的反应:密度泛函理论研究
1,2,4-三唑衍生物因其广泛的生物活性、良好的药效学和药代动力学特征以及低毒性而受到药物化学家的广泛关注,需要开发各种合成方法并对其进行全面研究。反应机制。 SMD/B3lyp 通过计算研究对新型螺缩合[1,2,4]三唑并[1,5-c]喹唑啉的形成可能途径进行了详细研究,该化合物结合了具有不同生物学特性的两个结构域/6-31+G(d)理论水平。 [2-(3-杂芳基-1,2,4-三唑-5-基)苯基]胺与环己酮在甲醇中的相互作用机制涉及三个主要过程: 通过胺与双键加成形成甲醇胺 C= O,消除水分子,分子内环化导致螺化合物的形成。结果表明,与非催化反应相比,酸催化反应过程中反应物的反应活性有所增加。三唑环上杂环取代基的性质对反应能量影响不大,但机理没有改变。































 京公网安备 11010802027423号
京公网安备 11010802027423号