当前位置:
X-MOL 学术
›
New J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Oxazoline amino acid bioconjugates: one-pot synthesis and analysis of supramolecular interactions
New Journal of Chemistry ( IF 2.7 ) Pub Date : 2024-04-05 , DOI: 10.1039/d4nj00995a Marija Bakija 1 , Berislav Perić 1 , Srećko I. Kirin 1
New Journal of Chemistry ( IF 2.7 ) Pub Date : 2024-04-05 , DOI: 10.1039/d4nj00995a Marija Bakija 1 , Berislav Perić 1 , Srećko I. Kirin 1
Affiliation
|
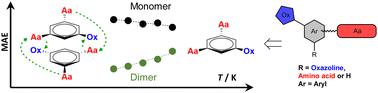
This publication describes oxazoline–amino acid bioconjugates 1 capable of supramolecular interactions. The bioconjugates contain three main building blocks: an oxazoline ring, a central aromatic unit and an amino acid substituent. Benzene, naphthalene or anthracene with several substitution motifs was used as the central aromatic unit. Two synthetic pathways to β-amino alcohol precursors 3–5 are presented; one-pot synthesis with various coupling reagents is compared to linearly sequenced synthesis using protecting groups. In the final step, a cyclization of precursors 3–5 to oxazolines 1 is described. Single crystal X-ray diffraction of seven oxazoline bioconjugates is reported (1p, 1m6, 1n2, 1n4, 1n5, 1a and 1t4), with an emphasis on supramolecular interactions in the solid state. The capability of bioconjugates 1 to participate in supramolecular interactions in solution was screened by NMR and CD spectroscopy, varying concentrations, temperatures and solvents. The results obtained by crystallography and spectroscopy were further corroborated by computational results for most interesting bioconjugate 1t1. Computational analysis was performed using a CREST/CENSO protocol. In particular, the free energy of formation, ΔG, as well as mean absolute error (MAE) values and correlations of experimental and GIAO calculated NMR parameters have been compared for 1t1 DFT models of monomers and dimers. These results revealed that for 1t1, the supramolecular dimer ensembles are more stable than monomer ensembles.
中文翻译:

恶唑啉氨基酸生物共轭物:一锅法合成及超分子相互作用分析
该出版物描述了能够进行超分子相互作用的恶唑啉-氨基酸生物缀合物1 。该生物结合物包含三个主要结构单元:恶唑啉环、中心芳香单元和氨基酸取代基。具有多个取代基的苯、萘或蒽被用作中心芳香单元。提出了β-氨基醇前体3 – 5的两种合成途径;将使用各种偶联试剂的一锅合成与使用保护基团的线性顺序合成进行比较。在最后一步中,描述了前体3 – 5环化为恶唑啉1 。报道了七种恶唑啉生物共轭物(1 p、1 m6、1 n2、1 n4、1 n5、1 a和1 t4 )的单晶X射线衍射,重点是固态下的超分子相互作用。通过 NMR 和 CD 光谱、不同浓度、温度和溶剂筛选生物缀合物1参与溶液中超分子相互作用的能力。最有趣的生物结合物1 t1的计算结果进一步证实了晶体学和光谱学获得的结果。使用 CREST/CENSO 协议进行计算分析。特别是,对于单体和二聚体的1 t1 DFT 模型,比较了形成自由能 Δ G以及平均绝对误差 (MAE) 值以及实验和 GIAO 计算的 NMR 参数的相关性。这些结果表明,对于1 t1,超分子二聚体整体比单体整体更稳定。
更新日期:2024-04-05
中文翻译:

恶唑啉氨基酸生物共轭物:一锅法合成及超分子相互作用分析
该出版物描述了能够进行超分子相互作用的恶唑啉-氨基酸生物缀合物1 。该生物结合物包含三个主要结构单元:恶唑啉环、中心芳香单元和氨基酸取代基。具有多个取代基的苯、萘或蒽被用作中心芳香单元。提出了β-氨基醇前体3 – 5的两种合成途径;将使用各种偶联试剂的一锅合成与使用保护基团的线性顺序合成进行比较。在最后一步中,描述了前体3 – 5环化为恶唑啉1 。报道了七种恶唑啉生物共轭物(1 p、1 m6、1 n2、1 n4、1 n5、1 a和1 t4 )的单晶X射线衍射,重点是固态下的超分子相互作用。通过 NMR 和 CD 光谱、不同浓度、温度和溶剂筛选生物缀合物1参与溶液中超分子相互作用的能力。最有趣的生物结合物1 t1的计算结果进一步证实了晶体学和光谱学获得的结果。使用 CREST/CENSO 协议进行计算分析。特别是,对于单体和二聚体的1 t1 DFT 模型,比较了形成自由能 Δ G以及平均绝对误差 (MAE) 值以及实验和 GIAO 计算的 NMR 参数的相关性。这些结果表明,对于1 t1,超分子二聚体整体比单体整体更稳定。








































 京公网安备 11010802027423号
京公网安备 11010802027423号