当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Electrochemical Reduction of Perfluorooctanoic Acid (PFOA): An Experimental and Theoretical Approach
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-04-05 , DOI: 10.1021/jacs.4c00443
Jonathan J Calvillo Solís 1, 2 , Christian Sandoval-Pauker 1, 2 , David Bai 1, 2 , Sheng Yin 1, 2 , Thomas P Senftle 2, 3 , Dino Villagrán 1, 2
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-04-05 , DOI: 10.1021/jacs.4c00443
Jonathan J Calvillo Solís 1, 2 , Christian Sandoval-Pauker 1, 2 , David Bai 1, 2 , Sheng Yin 1, 2 , Thomas P Senftle 2, 3 , Dino Villagrán 1, 2
Affiliation
|
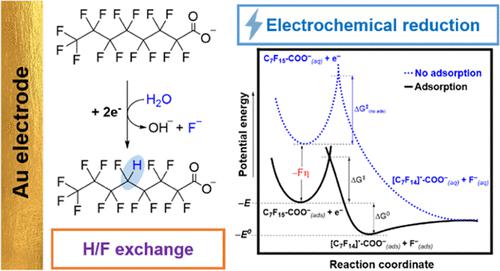
Perfluorooctanoic acid (PFOA) is an artificial chemical of global concern due to its high environmental persistence and potential human health risk. Electrochemical methods are promising technologies for water treatment because they are efficient, cheap, and scalable. The electrochemical reduction of PFOA is one of the current methodologies. This process leads to defluorination of the carbon chain to hydrogenated products. Here, we describe a mechanistic study of the electrochemical reduction of PFOA in gold electrodes. By using linear sweep voltammetry (LSV), an E0′ of −1.80 V vs Ag/AgCl was estimated. Using a scan rate diagnosis, we determined an electron-transfer coefficient (αexp) of 0.37, corresponding to a concerted mechanism. The strong adsorption of PFOA into the gold surface is confirmed by the Langmuir-like isotherm in the absence (KA = 1.89 × 1012 cm3 mol–1) and presence of a negative potential (KA = 3.94 × 107 cm3 mol–1, at −1.40 V vs Ag/AgCl). Based on Marcus–Hush’s theory, calculations show a solvent reorganization energy (λ0) of 0.9 eV, suggesting a large electrostatic repulsion between the perfluorinated chain and water. The estimated free energy of the transition state of the electron transfer (ΔG‡ = 2.42 eV) suggests that it is thermodynamically the reaction-limiting step. 19F – 1H NMR, UV–vis, and mass spectrometry studies confirm the displacement of fluorine atoms by hydrogen. Density functional theory (DFT) calculations also support the concerted mechanism for the reductive defluorination of PFOA, in agreement with the experimental values.
中文翻译:

全氟辛酸 (PFOA) 的电化学还原:实验和理论方法
全氟辛酸(PFOA)因其高环境持久性和潜在的人类健康风险而成为全球关注的人造化学品。电化学方法是有前途的水处理技术,因为它们高效、廉价且可扩展。 PFOA 的电化学还原是当前的方法之一。该过程导致碳链脱氟生成氢化产物。在这里,我们描述了金电极中 PFOA 电化学还原的机理研究。通过使用线性扫描伏安法(LSV),估计相对于Ag/AgCl的E 0 '为-1.80V。使用扫描速率诊断,我们确定电子转移系数 (α exp ) 为 0.37,对应于协调机制。在不存在负电势 ( KA = 1.89 × 10 12 cm 3 mol –1 ) 和存在负电势 ( K A = 3.94 × 10 7 cm 3 ) 的情况下,类 Langmuir 等温线证实了 PFOA 对金表面的强烈吸附mol –1 ,-1.40 V vs Ag/AgCl)。根据Marcus-Hush的理论,计算显示溶剂重组能(λ 0 )为0.9 eV,这表明全氟化链和水之间存在很大的静电斥力。电子转移过渡态的估计自由能 (Δ G ‡ = 2.42 eV) 表明它是热力学上的反应限制步骤。 19 F – 1 H NMR、UV-vis 和质谱研究证实氟原子被氢取代。 密度泛函理论 (DFT) 计算也支持 PFOA 还原脱氟的协同机制,与实验值一致。
更新日期:2024-04-05
中文翻译:

全氟辛酸 (PFOA) 的电化学还原:实验和理论方法
全氟辛酸(PFOA)因其高环境持久性和潜在的人类健康风险而成为全球关注的人造化学品。电化学方法是有前途的水处理技术,因为它们高效、廉价且可扩展。 PFOA 的电化学还原是当前的方法之一。该过程导致碳链脱氟生成氢化产物。在这里,我们描述了金电极中 PFOA 电化学还原的机理研究。通过使用线性扫描伏安法(LSV),估计相对于Ag/AgCl的E 0 '为-1.80V。使用扫描速率诊断,我们确定电子转移系数 (α exp ) 为 0.37,对应于协调机制。在不存在负电势 ( KA = 1.89 × 10 12 cm 3 mol –1 ) 和存在负电势 ( K A = 3.94 × 10 7 cm 3 ) 的情况下,类 Langmuir 等温线证实了 PFOA 对金表面的强烈吸附mol –1 ,-1.40 V vs Ag/AgCl)。根据Marcus-Hush的理论,计算显示溶剂重组能(λ 0 )为0.9 eV,这表明全氟化链和水之间存在很大的静电斥力。电子转移过渡态的估计自由能 (Δ G ‡ = 2.42 eV) 表明它是热力学上的反应限制步骤。 19 F – 1 H NMR、UV-vis 和质谱研究证实氟原子被氢取代。 密度泛函理论 (DFT) 计算也支持 PFOA 还原脱氟的协同机制,与实验值一致。

































 京公网安备 11010802027423号
京公网安备 11010802027423号