当前位置:
X-MOL 学术
›
Results Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
An expedient route for the synthesis of an anti-HIV peptide Kn2-7: A TAG approach
Results in Chemistry ( IF 2.5 ) Pub Date : 2024-03-29 , DOI: 10.1016/j.rechem.2024.101477 Akshitha D. Nagaraja , Veeranjaneyulu Avula , Selvakumar Balaraman , H. Surya Prakash Rao , Nagendra Govindappa
Results in Chemistry ( IF 2.5 ) Pub Date : 2024-03-29 , DOI: 10.1016/j.rechem.2024.101477 Akshitha D. Nagaraja , Veeranjaneyulu Avula , Selvakumar Balaraman , H. Surya Prakash Rao , Nagendra Govindappa
|
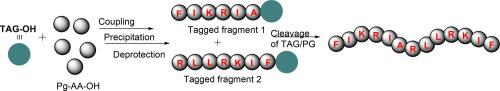
Human immunodeficiency virus (HIV) causes acquired immune deficiency syndrome (AIDS) where the immune system progressively deteriorates and consequently threatens life. The peptide Kn2-7, a modified version of scorpion venom non-disulphide peptide, possesses the highest anti-HIV activity inhibiting antiviral potencies of 13 HIV-1 strains. In the present study, we achieved a new synthesis of Kn2-7 through a commercially viable, scalable, and impurity-free approach using Tagging Technique. We used (2,4-bis(octadecyloxy)phenyl)methanol as the Tag support for the synthesis and couplings were achieved by COMU with Fmoc-protected amino acids. The method is highly advantageous over conventional SPPS or LPPS methods in terms of economy, time, and most importantly purification. The peptide Kn2-7 and the intermediates were isolated as pure solids, the structures of which were unequivocally confirmed by H NMR, C NMR, and Mass spectroscopy.
中文翻译:

合成抗 HIV 肽 Kn2-7 的便捷途径:TAG 方法
人类免疫缺陷病毒(HIV)会导致获得性免疫缺陷综合症(艾滋病),使免疫系统逐渐恶化,从而威胁生命。肽Kn2-7是蝎毒非二硫肽的改良版本,在13种HIV-1毒株中具有最高的抗HIV活性和抑制抗病毒效力。在本研究中,我们使用标记技术通过商业上可行的、可扩展的且无杂质的方法实现了 Kn2-7 的新合成。我们使用(2,4-双(十八烷氧基)苯基)甲醇作为合成的标签支持物,并通过 COMU 与 Fmoc 保护的氨基酸实现偶联。该方法在经济、时间和最重要的纯化方面比传统的 SPPS 或 LPPS 方法具有很大的优势。肽Kn2-7和中间体被分离为纯固体,其结构通过1H NMR、13C NMR和质谱明确证实。
更新日期:2024-03-29
中文翻译:

合成抗 HIV 肽 Kn2-7 的便捷途径:TAG 方法
人类免疫缺陷病毒(HIV)会导致获得性免疫缺陷综合症(艾滋病),使免疫系统逐渐恶化,从而威胁生命。肽Kn2-7是蝎毒非二硫肽的改良版本,在13种HIV-1毒株中具有最高的抗HIV活性和抑制抗病毒效力。在本研究中,我们使用标记技术通过商业上可行的、可扩展的且无杂质的方法实现了 Kn2-7 的新合成。我们使用(2,4-双(十八烷氧基)苯基)甲醇作为合成的标签支持物,并通过 COMU 与 Fmoc 保护的氨基酸实现偶联。该方法在经济、时间和最重要的纯化方面比传统的 SPPS 或 LPPS 方法具有很大的优势。肽Kn2-7和中间体被分离为纯固体,其结构通过1H NMR、13C NMR和质谱明确证实。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号