Beilstein Journal of Organic Chemistry ( IF 2.2 ) Pub Date : 2024-04-04 , DOI: 10.3762/bjoc.20.66 Senze Qiao 1 , Zhongyu Cheng 1 , Fuzhuo Li 1, 2
Abstract
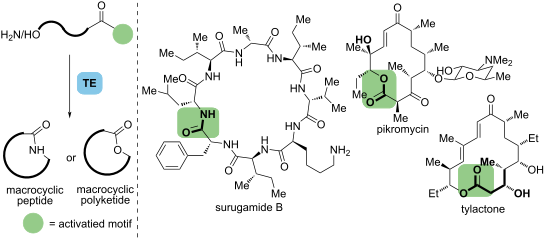
Chemoenzymatic strategies that combine synthetic and enzymatic transformations offer efficient approaches to yield target molecules, which have been increasingly employed in the synthesis of bioactive natural products. In the biosynthesis of macrocyclic nonribosomal peptides, polyketides, and their hybrids, thioesterase (TE) domains play a significant role in late-stage macrocyclization. These domains can accept mimics of native substrates in vitro and exhibit potential for use in total synthesis. This review summarizes the recent advances of TE domains in the chemoenzymatic synthesis for these natural products that aim to address the common issues in classical synthetic approaches and increase synthetic efficiencies, which have the potential to facilitate further pharmaceutical research.
Beilstein J. Org. Chem. 2024, 20, 721–733. doi:10.3762/bjoc.20.66
中文翻译:

通过硫酯酶催化大环化化学酶法合成大环肽和聚酮化合物
抽象的
结合合成和酶促转化的化学酶策略提供了产生目标分子的有效方法,这些分子已越来越多地用于生物活性天然产物的合成。在大环非核糖体肽、聚酮化合物及其混合物的生物合成中,硫酯酶 (TE) 结构域在后期大环化中发挥着重要作用。这些结构域可以在体外接受天然底物的模拟物,并表现出用于全合成的潜力。本综述总结了 TE 领域在这些天然产物的化学酶合成方面的最新进展,旨在解决经典合成方法中的常见问题并提高合成效率,这有可能促进进一步的药物研究。

Beilstein J. Org. Chem. 2024, 20, 721–733. doi:10.3762/bjoc.20.66































 京公网安备 11010802027423号
京公网安备 11010802027423号