当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
γ-Amino Alcohols via Energy Transfer Enabled Brook Rearrangement
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-04-03 , DOI: 10.1021/jacs.4c01667
Ranjini Laskar 1 , Subhabrata Dutta 1 , Jan C Spies 1 , Poulami Mukherjee 2 , Ángel Rentería-Gómez 2 , Rebecca E Thielemann 1 , Constantin G Daniliuc 1 , Osvaldo Gutierrez 2 , Frank Glorius 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-04-03 , DOI: 10.1021/jacs.4c01667
Ranjini Laskar 1 , Subhabrata Dutta 1 , Jan C Spies 1 , Poulami Mukherjee 2 , Ángel Rentería-Gómez 2 , Rebecca E Thielemann 1 , Constantin G Daniliuc 1 , Osvaldo Gutierrez 2 , Frank Glorius 1
Affiliation
|
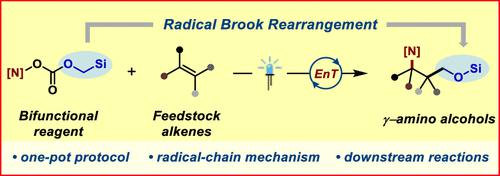
In the long-standing quest to synthesize fundamental building blocks with key functional group motifs, photochemistry in the recent past has comprehensively established its attractiveness. Amino alcohols are not only functionally diverse but are ubiquitous in the biologically active realm of compounds. We developed bench-stable bifunctional reagents that could then access the sparsely reported γ-amino alcohols directly from feedstock alkenes through energy transfer (EnT) photocatalysis. A designed 1,3-linkage across alkenes is made possible by the intervention of a radical Brook rearrangement that takes place downstream to the EnT-mediated homolysis of our reagent(s). A combination of experimental mechanistic investigations and detailed computational studies (DFT) indicates a radical chain propagated reaction pathway.
中文翻译:

通过能量转移实现布鲁克重排的 γ-氨基醇
在合成具有关键官能团基序的基本结构单元的长期探索中,光化学在最近已经全面确立了其吸引力。氨基醇不仅功能多样,而且在化合物的生物活性领域中普遍存在。我们开发了实验室稳定的双功能试剂,然后可以通过能量转移(EnT)光催化直接从原料烯烃中获取报道较少的γ-氨基醇。通过激进的布鲁克重排的干预,设计的跨烯烃 1,3-连接成为可能,该重排发生在我们的试剂的 EnT 介导的均裂下游。实验机制研究和详细计算研究(DFT)的结合表明了自由基链传播反应途径。
更新日期:2024-04-03
中文翻译:

通过能量转移实现布鲁克重排的 γ-氨基醇
在合成具有关键官能团基序的基本结构单元的长期探索中,光化学在最近已经全面确立了其吸引力。氨基醇不仅功能多样,而且在化合物的生物活性领域中普遍存在。我们开发了实验室稳定的双功能试剂,然后可以通过能量转移(EnT)光催化直接从原料烯烃中获取报道较少的γ-氨基醇。通过激进的布鲁克重排的干预,设计的跨烯烃 1,3-连接成为可能,该重排发生在我们的试剂的 EnT 介导的均裂下游。实验机制研究和详细计算研究(DFT)的结合表明了自由基链传播反应途径。































 京公网安备 11010802027423号
京公网安备 11010802027423号