当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Computational Insights into the Catalysis of the pH Dependence of Bromite Decomposition Catalyzed by Chlorite Dismutase from Dechloromonas aromatica (DaCld)
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2024-04-04 , DOI: 10.1021/acs.inorgchem.4c00126 Xianghui Zhang 1 , Yongjun Liu 1
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2024-04-04 , DOI: 10.1021/acs.inorgchem.4c00126 Xianghui Zhang 1 , Yongjun Liu 1
Affiliation
|
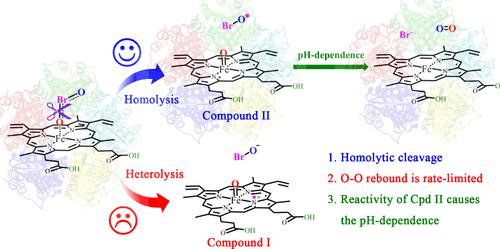
The heme-containing chlorite dismutases catalyze the rapid and efficient decomposition of chlorite (ClO2–) to yield Cl– and O2, and the catalytic efficiency of chlorite dismutase from Dechloromonas aromatica (DaCld) in catalyzing the decomposition of bromite (BrO2–) was dependent on pH, which was supposed to be caused by the conversion of active Cpd I to the inactive Cpd II by proton-coupled electron transfer (PCET) from the pocket Tyr118 to the propionate side chain of heme at high pH. However, the direct evidence of PCET and how the pH affects the efficiency of DaCld, as well as whether Cpd II is really inactive, are still poorly understood. Here, on the basis of the high-resolution crystal structures, the computational models in both acidic (pH 5.0) and alkaline (pH 9.0) environments were constructed, and a series of quantum mechanical/molecular mechanical calculations were performed. On the basis of our calculation results, the O–Br bond cleavage of BrO2– always follows the homolytic mode to generate Cpd II rather than Cpd I. It is different from the O–O cleavage of O2/H2O2 or peracetic acid catalyzed by the other heme-containing enzymes. Thus, in the subsequent O–O rebound reaction, it is the Fe(IV)═O in Cpd II that combines with the O–Br radical. Because the porphyrin ring in Cpd II does not bear an unpaired electron, the previously suggested PCET from Tyr118 to the propionate side chain of heme was not theoretically recognized in an alkaline environment. In addition, the O–O rebound step in an alkaline solution corresponds to an energy barrier that is larger than that in an acidic environment, which can well explain the pH dependence of the activity of DaCld. In addition, the protonation state of the propionic acid side chains of heme and the surrounding hydrogen bond networks were calculated to have a significant impact on the barriers of the O–O rebound step, which is mainly achieved by affecting the reactivity of the Fe(IV)═O group in Cpd II. In an acidic environment, the relatively weaker coordination of the O2 atom to Fe leads to its higher reactivity toward the O–O rebound reaction. These observations may provide useful information for understanding the catalysis of chlorite dismutases.
中文翻译:

芳香脱氯单胞菌 (DaCld) 亚氯酸盐歧化酶催化溴酸盐分解的 pH 依赖性的计算见解
含血红素的亚氯酸盐歧化酶催化亚氯酸盐(ClO 2 – )快速高效分解产生Cl –和O 2 ,以及来自芳香脱氯单胞菌( Da Cld)的亚氯酸盐歧化酶催化亚溴酸盐(BrO 2 )分解的催化效率– ) 取决于 pH 值,这被认为是由于在高 pH 条件下,通过质子耦合电子转移 (PCET) 从口袋 Tyr118 到血红素丙酸盐侧链,活性 Cpd I 转化为非活性 Cpd II 所致。然而,PCET 的直接证据以及 pH 如何影响Da Cld 的效率,以及 Cpd II 是否真的不活跃,仍然知之甚少。在此,基于高分辨率晶体结构,构建了酸性(pH 5.0)和碱性(pH 9.0)环境下的计算模型,并进行了一系列量子力学/分子力学计算。根据我们的计算结果,BrO 2 –的O-Br键断裂总是遵循均裂模式生成Cpd II而不是Cpd I。它与O 2 /H 2 O 2 或O 2 /H 2 O 2的O-O断裂不同。由其他含血红素的酶催化的过乙酸。因此,在随后的O-O反弹反应中,Cpd II中的Fe(IV)=O与O-Br自由基结合。由于 Cpd II 中的卟啉环不带有不成对电子,因此之前提出的从 Tyr118 到血红素丙酸侧链的 PCET 在碱性环境中理论上并未得到认可。 此外,碱性溶液中的O-O回弹步骤对应的能垒比酸性环境中的大,这可以很好地解释Da Cld活性的pH依赖性。此外,计算得出血红素丙酸侧链的质子化状态和周围的氢键网络对O-O反弹步骤的势垒有显着影响,这主要是通过影响Fe( IV)=化合物II中的O基团。在酸性环境中,O2原子与Fe的配位相对较弱,导致其对O-O反弹反应的反应性较高。这些观察结果可能为了解亚氯酸盐歧化酶的催化作用提供有用的信息。
更新日期:2024-04-04
中文翻译:

芳香脱氯单胞菌 (DaCld) 亚氯酸盐歧化酶催化溴酸盐分解的 pH 依赖性的计算见解
含血红素的亚氯酸盐歧化酶催化亚氯酸盐(ClO 2 – )快速高效分解产生Cl –和O 2 ,以及来自芳香脱氯单胞菌( Da Cld)的亚氯酸盐歧化酶催化亚溴酸盐(BrO 2 )分解的催化效率– ) 取决于 pH 值,这被认为是由于在高 pH 条件下,通过质子耦合电子转移 (PCET) 从口袋 Tyr118 到血红素丙酸盐侧链,活性 Cpd I 转化为非活性 Cpd II 所致。然而,PCET 的直接证据以及 pH 如何影响Da Cld 的效率,以及 Cpd II 是否真的不活跃,仍然知之甚少。在此,基于高分辨率晶体结构,构建了酸性(pH 5.0)和碱性(pH 9.0)环境下的计算模型,并进行了一系列量子力学/分子力学计算。根据我们的计算结果,BrO 2 –的O-Br键断裂总是遵循均裂模式生成Cpd II而不是Cpd I。它与O 2 /H 2 O 2 或O 2 /H 2 O 2的O-O断裂不同。由其他含血红素的酶催化的过乙酸。因此,在随后的O-O反弹反应中,Cpd II中的Fe(IV)=O与O-Br自由基结合。由于 Cpd II 中的卟啉环不带有不成对电子,因此之前提出的从 Tyr118 到血红素丙酸侧链的 PCET 在碱性环境中理论上并未得到认可。 此外,碱性溶液中的O-O回弹步骤对应的能垒比酸性环境中的大,这可以很好地解释Da Cld活性的pH依赖性。此外,计算得出血红素丙酸侧链的质子化状态和周围的氢键网络对O-O反弹步骤的势垒有显着影响,这主要是通过影响Fe( IV)=化合物II中的O基团。在酸性环境中,O2原子与Fe的配位相对较弱,导致其对O-O反弹反应的反应性较高。这些观察结果可能为了解亚氯酸盐歧化酶的催化作用提供有用的信息。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号