当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
First-Generation Process Development for the Synthesis of Baloxavir Marboxil: Early-Stage Development of Synthetic Methods to Prepare Baloxavir Marboxil Intermediates
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2024-04-03 , DOI: 10.1021/acs.oprd.3c00514 Kosuke Anan 1 , Masayoshi Miyagawa 1 , Azusa Okano 1 , Hideki Sugimoto 1 , Naoki Miyake 1 , Nobuaki Fukui 2 , Akihito Kijima 2 , Emi Tanimoto 2 , Makoto Kawai 1
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2024-04-03 , DOI: 10.1021/acs.oprd.3c00514 Kosuke Anan 1 , Masayoshi Miyagawa 1 , Azusa Okano 1 , Hideki Sugimoto 1 , Naoki Miyake 1 , Nobuaki Fukui 2 , Akihito Kijima 2 , Emi Tanimoto 2 , Makoto Kawai 1
Affiliation
|
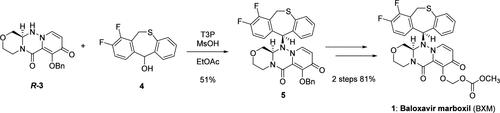
Described herein is the discovery and development of a process to prepare chiral triazinanone R-3 and diastereomeric intermediate 5, the key intermediates in the synthesis of the cap-dependent endonuclease inhibitor baloxavir marboxil (1), which can suppress the replication of influenza virus. Chiral triazinanone R-3 was obtained via optical resolution of its racemic form rac-3. Diastereomeric intermediate 5 was obtained by the condensation reaction of triazinanone R-3 and thiepin alcohol 4 using a combination of T3P and MsOH. These reactions were performed successfully on kilogram scale and were critical to the establishment of the baloxavir marboxil manufacturing process.
中文翻译:

Baloxavir Marboxil 合成的第一代工艺开发:制备 Baloxavir Marboxil 中间体的合成方法的早期开发
本文描述的是制备手性三嗪酮 R-3 和非对映异构体中间体 5 的方法的发现和开发,这是合成帽依赖性核酸内切酶抑制剂 baloxavir marboxil (1) 的关键中间体,可抑制流感病毒的复制。手性三嗪酮 R-3 通过光学拆分其外消旋形式 rac-3 获得。使用T3P和MsOH的组合,通过三嗪酮R-3和硫杂苯醇4的缩合反应得到非对映体中间体5。这些反应在公斤级上成功进行,对于巴洛沙韦马波西生产工艺的建立至关重要。
更新日期:2024-04-03
中文翻译:

Baloxavir Marboxil 合成的第一代工艺开发:制备 Baloxavir Marboxil 中间体的合成方法的早期开发
本文描述的是制备手性三嗪酮 R-3 和非对映异构体中间体 5 的方法的发现和开发,这是合成帽依赖性核酸内切酶抑制剂 baloxavir marboxil (1) 的关键中间体,可抑制流感病毒的复制。手性三嗪酮 R-3 通过光学拆分其外消旋形式 rac-3 获得。使用T3P和MsOH的组合,通过三嗪酮R-3和硫杂苯醇4的缩合反应得到非对映体中间体5。这些反应在公斤级上成功进行,对于巴洛沙韦马波西生产工艺的建立至关重要。































 京公网安备 11010802027423号
京公网安备 11010802027423号