当前位置:
X-MOL 学术
›
Sci. Total Environ.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Computational-aided analysis of the pathway and mechanism of dichloroacetonitrile formation from phenylalanine upon chloramination
Science of the Total Environment ( IF 8.2 ) Pub Date : 2024-03-26 , DOI: 10.1016/j.scitotenv.2024.171995 Rui Yu 1 , Yunkun Qian 1 , Yanan Chen 1 , Yijun Shi 1 , Jun Guo 1 , Dong An 2
Science of the Total Environment ( IF 8.2 ) Pub Date : 2024-03-26 , DOI: 10.1016/j.scitotenv.2024.171995 Rui Yu 1 , Yunkun Qian 1 , Yanan Chen 1 , Yijun Shi 1 , Jun Guo 1 , Dong An 2
Affiliation
|
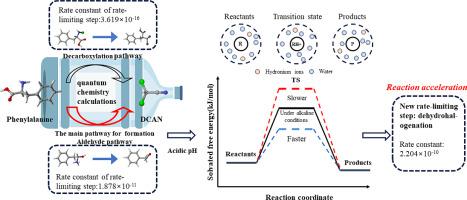
Dichloroacetonitrile (DCAN) is an emerging disinfection by-product (DBP) that is widespread in drinking water. However, the pathway for DCAN formation from aromatic amino acids remains unclear, leading to a lack of an understanding of its explicit fate during chloramination. In this study, we investigated the specific formation mechanism of DCAN during the chloramination of phenylalanine based on reaction kinetics and chemical thermodynamics. The reason for differences between aldehyde and decarboxylation pathways was explained, and kinetic parameters of the pathways were obtained through quantum chemistry calculations. The results showed that the reaction rate constant of the rate-limiting step of the aldehyde pathway with 1.9 × 10−11 s−1 was significantly higher than that of decarboxylation (3.6 × 10−16 s−1 M−1 ), suggesting that the aldehyde pathway is the main reaction pathway for DCAN formation during the chloramination of phenylalanine to produce DCAN. Subsequently, theoretical calculations were performed to elucidate the effect of pH on the formation mechanism, which aligned well with the experimental results. Dehydrohalogenation was found to be the rate-limiting step under acidic conditions with reaction rate constants higher than those of the rate-limiting step (expulsion of amines) under neutral conditions, increasing the rate of DCAN formation. This study highlights the differences in DCAN formation between the decarboxylation and aldehyde pathways during the chloramination of precursors at both molecular and kinetic levels, contributing to a comprehensive understanding of the reaction mechanisms by which aromatic free amino acids generate DCAN.
中文翻译:

氯胺化后苯丙氨酸形成二氯乙腈的途径和机制的计算辅助分析
二氯乙腈 (DCAN) 是一种新兴的消毒副产物 (DBP),广泛存在于饮用水中。然而,芳香族氨基酸形成 DCAN 的途径仍不清楚,导致对其在氯胺化过程中的明确命运缺乏了解。在本研究中,我们基于反应动力学和化学热力学研究了苯丙氨酸氯胺化过程中 DCAN 的具体形成机制。解释了醛和脱羧途径之间存在差异的原因,并通过量子化学计算获得了途径的动力学参数。结果表明,醛途径限速步骤的反应速率常数为 1.9 × 10-11 s-1 显著高于脱羧(3.6 × 10-16 s-1 M-1),表明醛途径是苯丙氨酸氯胺化生成 DCAN 过程中形成 DCAN 的主要反应途径。随后,进行了理论计算以阐明 pH 值对形成机制的影响,这与实验结果非常吻合。发现脱卤化氢在酸性条件下是限速步骤,反应速率常数高于中性条件下限速步骤(胺的排出),从而提高了 DCAN 形成的速率。本研究强调了前体氯胺化过程中脱羧和醛途径之间 DCAN 形成的分子和动力学水平的差异,有助于全面了解芳香族游离氨基酸生成 DCAN 的反应机制。
更新日期:2024-03-26
中文翻译:

氯胺化后苯丙氨酸形成二氯乙腈的途径和机制的计算辅助分析
二氯乙腈 (DCAN) 是一种新兴的消毒副产物 (DBP),广泛存在于饮用水中。然而,芳香族氨基酸形成 DCAN 的途径仍不清楚,导致对其在氯胺化过程中的明确命运缺乏了解。在本研究中,我们基于反应动力学和化学热力学研究了苯丙氨酸氯胺化过程中 DCAN 的具体形成机制。解释了醛和脱羧途径之间存在差异的原因,并通过量子化学计算获得了途径的动力学参数。结果表明,醛途径限速步骤的反应速率常数为 1.9 × 10-11 s-1 显著高于脱羧(3.6 × 10-16 s-1 M-1),表明醛途径是苯丙氨酸氯胺化生成 DCAN 过程中形成 DCAN 的主要反应途径。随后,进行了理论计算以阐明 pH 值对形成机制的影响,这与实验结果非常吻合。发现脱卤化氢在酸性条件下是限速步骤,反应速率常数高于中性条件下限速步骤(胺的排出),从而提高了 DCAN 形成的速率。本研究强调了前体氯胺化过程中脱羧和醛途径之间 DCAN 形成的分子和动力学水平的差异,有助于全面了解芳香族游离氨基酸生成 DCAN 的反应机制。






























 京公网安备 11010802027423号
京公网安备 11010802027423号