当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Comparative Study on the H-Abstraction Reactions of Isopropyl Acetate and Propyl Acetate by HO2 and OH Radicals
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2024-04-02 , DOI: 10.1021/acs.jpca.4c00794 Mengjiao Gao 1 , Jiuning He 1 , Lilan Tian 1 , Lei Chen 1 , Shunping Shi 1 , Changhua Zhang 2 , Deliang Chen 3
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2024-04-02 , DOI: 10.1021/acs.jpca.4c00794 Mengjiao Gao 1 , Jiuning He 1 , Lilan Tian 1 , Lei Chen 1 , Shunping Shi 1 , Changhua Zhang 2 , Deliang Chen 3
Affiliation
|
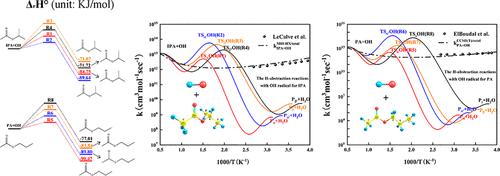
Isopropyl acetate (IPA) and propyl acetate (PA) are recognized as promising biofuels suitable for applications as fuel additives and biodiesel models. The H-abstraction reactions with radicals stand out as the fundamental initiating reactions in the combustion kinetic models for IPA and PA. In the present work, the kinetic calculations of IPA and PA plus HO2 and OH radicals were investigated at M06-2X/cc-pVTZ//G4, M08-HX/maug-cc-pVTZ, and CCSD(T)/jul-cc-pVTZ levels. The thermodynamic calculations were obtained based on the G4 and CBS-APNO methods. Rate coefficients were calculated using both transition state theory and canonical variational transition state theory with tunneling correction at the temperature range of 250–2000 K. The total rate constants for the IPA + OH system were fitted as follows: k = 0.4674 × T3.927 exp(2128/T) (cm3 mol–1 s–1), and for the PA + OH system, the total rate constants were determined using the following equation: k = 0.0161 × T4.373 exp(2220/T) (cm3 mol–1 s–1). The rate coefficients of IPA + OH reactions determined based on the M08-HX/maug-cc-pVTZ level effectively replicate the experimental data, while H-abstraction rate coefficients of PA + OH by the CCSD(T)/jul-cc-pVTZ method accurately reproduce the experimental data. Refining the H-abstraction rate coefficients in the kinetic mechanism of PA, as proposed by Dayma et al. [Proc. Combust. Inst. 37 (2019) 429–436], has been achieved through incorporating the present calculated data, leading to the development of a revised mechanism. The validation of the updated mechanism against jet-stirred reactor data is presented, showcasing its effective performance in predicting JSR data.
中文翻译:

HO2和OH自由基对乙酸异丙酯和乙酸丙酯的夺氢反应的比较研究
乙酸异丙酯 (IPA) 和乙酸丙酯 (PA) 被认为是有前途的生物燃料,适合用作燃料添加剂和生物柴油模型。与自由基的 H 抽象反应是 IPA 和 PA 燃烧动力学模型中的基本引发反应。在目前的工作中,IPA 和 PA 加上 HO 2和 OH 自由基的动力学计算在 M06-2X/cc-pVTZ//G4、M08-HX/maug-cc-pVTZ 和 CCSD(T)/jul- 上进行了研究。 cc-pVTZ 水平。热力学计算是基于G4和CBS-APNO方法获得的。在 250–2000 K 的温度范围内,使用过渡态理论和典型变分过渡态理论以及隧道校正计算速率系数。IPA + OH 系统的总速率常数拟合如下: k = 0.4674 × T 3.927 exp (2128/ T ) (cm 3 mol –1 s –1 ),对于 PA + OH 体系,总速率常数使用以下方程确定: k = 0.0161 × T 4.373 exp(2220/ T ) (cm 3摩尔–1秒–1 )。基于M08-HX/maug-cc-pVTZ水平确定的IPA + OH反应速率系数有效地复制了实验数据,而CCSD(T)/jul-cc-pVTZ水平确定的PA + OH H-抽象速率系数方法准确地再现了实验数据。 Dayma 等人提出,完善 PA 动力学机制中的 H 抽象速率系数。 [程序。燃烧。研究所。 37 (2019) 429–436],是通过合并现有计算数据实现的,从而制定了修订后的机制。 更新的机制针对喷射搅拌反应器数据进行了验证,展示了其在预测 JSR 数据方面的有效性能。
更新日期:2024-04-02
中文翻译:

HO2和OH自由基对乙酸异丙酯和乙酸丙酯的夺氢反应的比较研究
乙酸异丙酯 (IPA) 和乙酸丙酯 (PA) 被认为是有前途的生物燃料,适合用作燃料添加剂和生物柴油模型。与自由基的 H 抽象反应是 IPA 和 PA 燃烧动力学模型中的基本引发反应。在目前的工作中,IPA 和 PA 加上 HO 2和 OH 自由基的动力学计算在 M06-2X/cc-pVTZ//G4、M08-HX/maug-cc-pVTZ 和 CCSD(T)/jul- 上进行了研究。 cc-pVTZ 水平。热力学计算是基于G4和CBS-APNO方法获得的。在 250–2000 K 的温度范围内,使用过渡态理论和典型变分过渡态理论以及隧道校正计算速率系数。IPA + OH 系统的总速率常数拟合如下: k = 0.4674 × T 3.927 exp (2128/ T ) (cm 3 mol –1 s –1 ),对于 PA + OH 体系,总速率常数使用以下方程确定: k = 0.0161 × T 4.373 exp(2220/ T ) (cm 3摩尔–1秒–1 )。基于M08-HX/maug-cc-pVTZ水平确定的IPA + OH反应速率系数有效地复制了实验数据,而CCSD(T)/jul-cc-pVTZ水平确定的PA + OH H-抽象速率系数方法准确地再现了实验数据。 Dayma 等人提出,完善 PA 动力学机制中的 H 抽象速率系数。 [程序。燃烧。研究所。 37 (2019) 429–436],是通过合并现有计算数据实现的,从而制定了修订后的机制。 更新的机制针对喷射搅拌反应器数据进行了验证,展示了其在预测 JSR 数据方面的有效性能。































 京公网安备 11010802027423号
京公网安备 11010802027423号