当前位置:
X-MOL 学术
›
New J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis, biological activity, and binding modes of novel guanidino-containing neonicotinoid derivatives
New Journal of Chemistry ( IF 2.7 ) Pub Date : 2024-04-01 , DOI: 10.1039/d4nj00228h Jianyang Li 1 , Jing Miao 1 , Peibo Liang 1 , Yiyang Wang 1 , Xingyue Zhou 1 , Huizhe Lu 1 , Yanhong Dong 1 , Jianjun Zhang 1
New Journal of Chemistry ( IF 2.7 ) Pub Date : 2024-04-01 , DOI: 10.1039/d4nj00228h Jianyang Li 1 , Jing Miao 1 , Peibo Liang 1 , Yiyang Wang 1 , Xingyue Zhou 1 , Huizhe Lu 1 , Yanhong Dong 1 , Jianjun Zhang 1
Affiliation
|
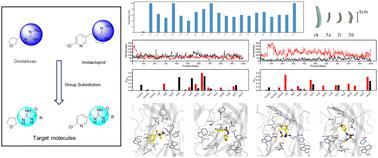
In the present study, the guanidine moiety from natural products was introduced into neonicotinoid insecticides, and a series of nicotine derivatives with guanidine functional groups were synthesized. Bioactivity assays reveal that compounds 7a and 7i, despite having significant structural differences, both exhibit notable insecticidal activity. Molecular docking, MD simulations, and aNCI results indicate different binding modes between nAChR and the compounds, with 7a exhibiting predominantly hydrophobic interactions and 7i showing dominance in hydrogen bonding interactions. The analysis concluded that using cyclic structures for structural derivation may be a more efficient strategy for designing inhibitors of nAChR.
中文翻译:

新型含胍基新烟碱类衍生物的合成、生物活性和结合模式
本研究将天然产物中的胍基团引入新烟碱类杀虫剂中,合成了一系列带有胍官能团的烟碱衍生物。生物活性测定表明,化合物7a和7i尽管具有显着的结构差异,但均表现出显着的杀虫活性。分子对接、MD 模拟和 aNCI 结果表明 nAChR 和化合物之间存在不同的结合模式,其中7a主要表现出疏水性相互作用,而7i显示出氢键相互作用占主导地位。分析得出结论,使用环状结构进行结构衍生可能是设计 nAChR 抑制剂的更有效策略。
更新日期:2024-04-01
中文翻译:

新型含胍基新烟碱类衍生物的合成、生物活性和结合模式
本研究将天然产物中的胍基团引入新烟碱类杀虫剂中,合成了一系列带有胍官能团的烟碱衍生物。生物活性测定表明,化合物7a和7i尽管具有显着的结构差异,但均表现出显着的杀虫活性。分子对接、MD 模拟和 aNCI 结果表明 nAChR 和化合物之间存在不同的结合模式,其中7a主要表现出疏水性相互作用,而7i显示出氢键相互作用占主导地位。分析得出结论,使用环状结构进行结构衍生可能是设计 nAChR 抑制剂的更有效策略。































 京公网安备 11010802027423号
京公网安备 11010802027423号