当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Rapid and Modular Access to Multifunctionalized 1,2-Azaborines via Palladium/Norbornene Cooperative Catalysis
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-03-29 , DOI: 10.1021/jacs.4c01582
Shinyoung Choi 1 , Guangbin Dong 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-03-29 , DOI: 10.1021/jacs.4c01582
Shinyoung Choi 1 , Guangbin Dong 1
Affiliation
|
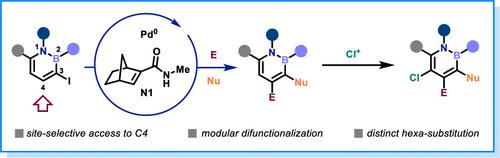
1,2-Azaborines, a unique class of BN-isosteres of benzene, have attracted great interest across several fields. While significant advancements have been made in the postfunctionalization of 1,2-azaborines, challenges still exist for the selective functionalization of the C4 position and access to 1,2-azaborines with five or six independently installed substituents. Here we report a rapid and modular method for C3 and C4 difunctionalization of 1,2-azaborines using the palladium/norbornene (Pd/NBE) cooperative catalysis. Enabled by the C2 amide-substituted NBE, diverse 3-iodo-1,2-azaborines can be used as substrates, showing broad functional group tolerance. Besides ortho arylation, preliminary success of ortho alkylation has also been realized. In addition, a range of alkenes and nucleophiles can be employed for ipso C3 functionalization. The reaction is scalable, and various postfunctionalizations, including forming hexa-substituted 1,2-azaborines, have been achieved.
中文翻译:

通过钯/降冰片烯协同催化快速、模块化地获得多功能 1,2-氮杂硼烯
1,2-氮杂硼是苯的一类独特的 BN 等排体,在多个领域引起了极大的兴趣。虽然 1,2-氮杂蛋白的后功能化取得了重大进展,但 C4 位的选择性官能化和获得具有 5 个或 6 个独立安装的取代基的 1,2-氮杂蛋白仍然存在挑战。在这里,我们报道了一种使用钯/降冰片烯 (Pd/NBE) 协同催化对 1,2-氮杂霉烯进行 C3 和 C4 二官能化的快速模块化方法。在 C2 酰胺取代的 NBE 的支持下,多种 3-碘-1,2-氮杂硼酸可用作底物,显示出广泛的官能团耐受性。除了邻位芳基化,邻位烷基化也取得了初步成功。此外,一系列烯烃和亲核试剂可用于 ipso C3 官能团化。该反应是可扩展的,并且已经实现了各种后功能化,包括形成六取代的 1,2-氮杂花硼酸。
更新日期:2024-03-29
中文翻译:

通过钯/降冰片烯协同催化快速、模块化地获得多功能 1,2-氮杂硼烯
1,2-氮杂硼是苯的一类独特的 BN 等排体,在多个领域引起了极大的兴趣。虽然 1,2-氮杂蛋白的后功能化取得了重大进展,但 C4 位的选择性官能化和获得具有 5 个或 6 个独立安装的取代基的 1,2-氮杂蛋白仍然存在挑战。在这里,我们报道了一种使用钯/降冰片烯 (Pd/NBE) 协同催化对 1,2-氮杂霉烯进行 C3 和 C4 二官能化的快速模块化方法。在 C2 酰胺取代的 NBE 的支持下,多种 3-碘-1,2-氮杂硼酸可用作底物,显示出广泛的官能团耐受性。除了邻位芳基化,邻位烷基化也取得了初步成功。此外,一系列烯烃和亲核试剂可用于 ipso C3 官能团化。该反应是可扩展的,并且已经实现了各种后功能化,包括形成六取代的 1,2-氮杂花硼酸。

































 京公网安备 11010802027423号
京公网安备 11010802027423号