当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Rh(III)-Catalyzed Diastereo- and Enantioselective Regiodivergent (Hetero)Arylamidation of (Homo)Allylic Sulfides
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-03-28 , DOI: 10.1021/jacs.3c14041
Xiaoyan Jia 1 , Gui-Lin Hao 2 , Mengxia Feng 1 , Huanfeng Jiang 1 , Shou-Guo Wang 2 , Liangbin Huang 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-03-28 , DOI: 10.1021/jacs.3c14041
Xiaoyan Jia 1 , Gui-Lin Hao 2 , Mengxia Feng 1 , Huanfeng Jiang 1 , Shou-Guo Wang 2 , Liangbin Huang 1
Affiliation
|
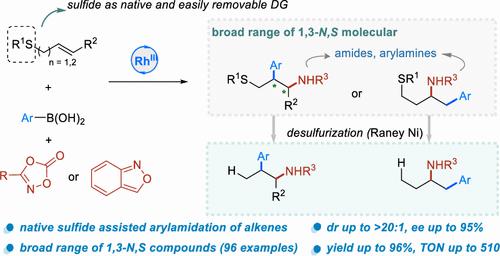
A rhodium-catalyzed 3-component conjunctive diastereo- and regioselective arylamidation of (homo)allylic sulfides, organon boronic acids, and dioxazolones is reported. These reactions deliver the 1,2-insertion and 2,1-insertion arylamidation products, respectively, for allylic sulfides and homoallylic sulfides. The enantioselective arylamidation of terminal and internal allylic sulfides is achieved, furnishing various 1,3-N,S compounds featuring one or two contiguous stereocenters in high yields and with high diastereo- and enantioselectivities. Mechanistic studies suggest a change in the turnover-limiting and selectivity-determining steps induced by the native and easily removable sulfide group.
中文翻译:

Rh(III) 催化的(同)烯丙基硫醚的非对映和对映选择性区域发散(杂)芳基酰胺化
报道了(同)烯丙基硫化物、有机硼酸和二恶唑酮的铑催化三组分联合非对映和区域选择性芳基酰胺化。这些反应分别产生烯丙基硫化物和高烯丙基硫化物的 1,2-插入和 2,1-插入芳基酰胺化产物。实现了末端和内部烯丙基硫化物的对映选择性芳基酰胺化,以高产率和高非对映选择性和对映选择性提供具有一个或两个连续立构中心的各种1,3- N,S化合物。机理研究表明,由天然且易于去除的硫化物基团引起的周转限制和选择性决定步骤发生了变化。
更新日期:2024-03-28
中文翻译:

Rh(III) 催化的(同)烯丙基硫醚的非对映和对映选择性区域发散(杂)芳基酰胺化
报道了(同)烯丙基硫化物、有机硼酸和二恶唑酮的铑催化三组分联合非对映和区域选择性芳基酰胺化。这些反应分别产生烯丙基硫化物和高烯丙基硫化物的 1,2-插入和 2,1-插入芳基酰胺化产物。实现了末端和内部烯丙基硫化物的对映选择性芳基酰胺化,以高产率和高非对映选择性和对映选择性提供具有一个或两个连续立构中心的各种1,3- N,S化合物。机理研究表明,由天然且易于去除的硫化物基团引起的周转限制和选择性决定步骤发生了变化。































 京公网安备 11010802027423号
京公网安备 11010802027423号