当前位置:
X-MOL 学术
›
Food Biosci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Research on the screening and inhibition mechanism of angiotensin I-converting enzyme (ACE) inhibitory peptides from tuna dark muscle
Food Bioscience ( IF 4.8 ) Pub Date : 2024-03-24 , DOI: 10.1016/j.fbio.2024.103956
Xin-Yu Zu , Ya-Nan Zhao , Yan Liang , Ying-Qiu Li , Chen-Ying Wang , Xiang-Zhong Zhao , Hua Wang
Food Bioscience ( IF 4.8 ) Pub Date : 2024-03-24 , DOI: 10.1016/j.fbio.2024.103956
Xin-Yu Zu , Ya-Nan Zhao , Yan Liang , Ying-Qiu Li , Chen-Ying Wang , Xiang-Zhong Zhao , Hua Wang
|
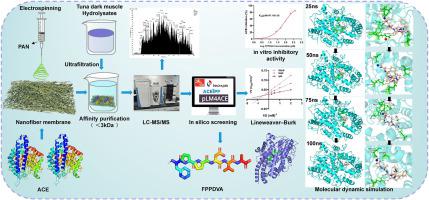
The rapid screening of angiotensin I-converting enzyme (ACE) inhibitory peptides (ACEIPs) from protein hydrolysates remains a challenge in current researches. The current study presents the development of an efficient affinity medium (AOPAN–ACE) consisting of ACE immobilized on the surface of electrostatically spun polyacrylonitrile nanofibrous membranes for the efficient screening of ACEIPs. The application of this affinity medium followed by liquid chromatography–tandem mass spectrometry analysis resulted in the successful screening and identification of 60 potential ACEIPs from protein hydrolysates of tuna dark muscle. Notably, the application of PeptideRanker, pLM4ACE, and molecular docking approaches resulted in the screening and identification of a novel ACEIP FPPDVA. Furthermore, the peptide FPPDVA was synthesized using the solid-phase method, and its IC50 value on ACE was determined to be 87.11 ± 1.02 μM. Molecular docking analysis suggested that the ACEIP of the peptide FPPDVA is attributable to the formation of hydrogen bonds with the S1 active site (Ala354 and Tyr523) of ACE and metal–ligand bonds with Zn2+ . In addition, molecular dynamics simulations revealed that the peptide FPPDVA formed a stable complex with ACE and was consistently localized within the active pocket of ACE over a simulation duration of 100 ns without being displaced from the active site. Lineweaver–Burk analysis further confirmed that the peptide FPPDVA exhibited mixed mode of inhibition against ACE. Overall, the affinity medium developed in the current study is potentially useful for highly efficient screening of ACEIPs, and the novel ACEIP FPPDVA identified herein is a promising functional food ingredient for antihypertensive application.
中文翻译:

金枪鱼深色肌肉血管紧张素 I 转换酶 (ACE) 抑制肽的筛选及抑制机制研究
从蛋白质水解物中快速筛选血管紧张素 I 转换酶 (ACE) 抑制肽 (ACEIPs) 仍然是当前研究的一个挑战。目前的研究提出了一种高效亲和介质 (AOPAN-ACE) 的开发,该介质由固定在静电纺丝聚丙烯腈纳米纤维膜表面的 ACE 组成,用于有效筛选 ACEIPs。应用这种亲和介质,然后进行液相色谱-串联质谱分析,成功筛选和鉴定了金枪鱼深色肌肉蛋白质水解物中的 60 种潜在 ACEIP。值得注意的是,PeptideRanker、pLM4ACE 和分子对接方法的应用导致了新型 ACEIP FPPDVA 的筛选和鉴定。此外,采用固相法合成肽 FPPDVA,其对 ACE 的 IC50 值为 87.11 ± 1.02 μM。分子对接分析表明,肽 FPPDVA 的 ACEIP 归因于与 ACE 的 S1 活性位点(Ala354 和 Tyr523)形成氢键以及与 Zn2+ 的金属-配体键。此外,分子动力学模拟显示,肽 FPPDVA 与 ACE 形成稳定的复合物,并在 100 ns 的模拟持续时间内始终定位在 ACE 的活性口袋内,而不会从活性位点移位。Lineweaver-Burk 分析进一步证实肽 FPPDVA 表现出对 ACE 的混合抑制模式。总体而言,本研究中开发的亲和培养基可能可用于 ACEIPs 的高效筛选,并且本文确定的新型 ACEIP FPPDVA 是一种很有前途的抗高血压应用功能性食品成分。
更新日期:2024-03-24
中文翻译:

金枪鱼深色肌肉血管紧张素 I 转换酶 (ACE) 抑制肽的筛选及抑制机制研究
从蛋白质水解物中快速筛选血管紧张素 I 转换酶 (ACE) 抑制肽 (ACEIPs) 仍然是当前研究的一个挑战。目前的研究提出了一种高效亲和介质 (AOPAN-ACE) 的开发,该介质由固定在静电纺丝聚丙烯腈纳米纤维膜表面的 ACE 组成,用于有效筛选 ACEIPs。应用这种亲和介质,然后进行液相色谱-串联质谱分析,成功筛选和鉴定了金枪鱼深色肌肉蛋白质水解物中的 60 种潜在 ACEIP。值得注意的是,PeptideRanker、pLM4ACE 和分子对接方法的应用导致了新型 ACEIP FPPDVA 的筛选和鉴定。此外,采用固相法合成肽 FPPDVA,其对 ACE 的 IC50 值为 87.11 ± 1.02 μM。分子对接分析表明,肽 FPPDVA 的 ACEIP 归因于与 ACE 的 S1 活性位点(Ala354 和 Tyr523)形成氢键以及与 Zn2+ 的金属-配体键。此外,分子动力学模拟显示,肽 FPPDVA 与 ACE 形成稳定的复合物,并在 100 ns 的模拟持续时间内始终定位在 ACE 的活性口袋内,而不会从活性位点移位。Lineweaver-Burk 分析进一步证实肽 FPPDVA 表现出对 ACE 的混合抑制模式。总体而言,本研究中开发的亲和培养基可能可用于 ACEIPs 的高效筛选,并且本文确定的新型 ACEIP FPPDVA 是一种很有前途的抗高血压应用功能性食品成分。


































 京公网安备 11010802027423号
京公网安备 11010802027423号