Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
An Exploratory Experimental Analysis Backed by Quantum Mechanical Modeling, Spectroscopic, and Surface Study for C-Steel Surface in the Presence of Hydrazone-Based Schiff Bases to Fix Corrosion Defects in Acidic Media
ACS Omega ( IF 3.7 ) Pub Date : 2024-03-28 , DOI: 10.1021/acsomega.4c00199 Amira H. E. Moustafa 1 , Hanaa H. Abdel-Rahman 1 , Assem Barakat 2 , Hagar A. Mohamed 3 , Ahmed S. El-Kholany 3
ACS Omega ( IF 3.7 ) Pub Date : 2024-03-28 , DOI: 10.1021/acsomega.4c00199 Amira H. E. Moustafa 1 , Hanaa H. Abdel-Rahman 1 , Assem Barakat 2 , Hagar A. Mohamed 3 , Ahmed S. El-Kholany 3
Affiliation
|
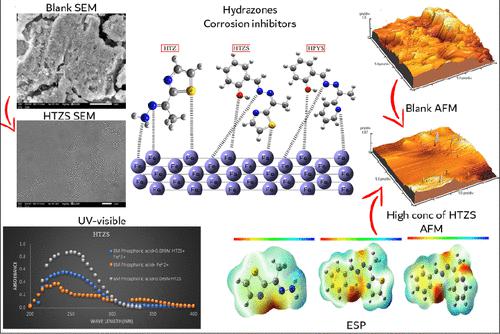
This work focuses on developing corrosion control and protecting the environment by creating affordable, sustainable, environmentally friendly, and efficient corrosion resistance chemicals. That is, through synthesized three hydrazone Schiff bases E-2-(1-hydrazonoethyl)thiazole (HTZ), 2-((E)-(((Z)-1-(thiazol-2-yl)ethylidene)hydrazono)methyl)phenol (HTZS), and 2-((E)-(((Z)-1-(pyridin-2-yl)ethylidene)hydrazono)methyl)phenol (HPYS) and corrosion inhibitors for C-steel in 8 M H3PO4 solution that were studied. The chemicals were analyzed by using 1H NMR and 13C NMR spectroscopy to learn more about them. Predominantly, the hydrazone-based Schiff bases have been considered powerful inhibitors due to their ability to be adsorbed with very low concentrations through their reactive sites (N, O, and S). Maximum surface (θmax) coverage and inhibition efficiency of 83.33% were sufficiently found at 99.00 × 10–3 mol/L concentration of HTZS at 293 K. Galvanostatic experiments demonstrated that raising the concentration of hydrazones improved mass transfer resistance. To study microstructure, scanning, reflectance, and energy-dispersive X-rays were used. Roughness and qualitative adhesion of the adsorbed layer were estimated by an atomic force microscope. After adding 99.00 × 10–3 mol/L of HTZS, the degree of surface brightness and reflectance increases to 137.20, relative to the corroded electrolyte-free solution 27.70. The roughness (Ra) decreased from 0.468 to 0.088 μm by adding HTZS. A surface morphology study confirmed that adding hydrazones to the C-steel dissolution bath greatly improves the surface’s look and texture quality. The atomic absorption spectroscopy technique was used to compare the concentration of the iron ions that remained in the solution after galvanostatic analysis in the absence and presence of the hydrazones under different conditions; it was found that the inhibited solution contained lower concentrations of iron ions as compared to the uninhibited solution. The DFT theoretical analysis verified the observation of hydrazone physical adsorption through bonding electrons that obey kinetic adsorption isotherms. It is based on examining the highest occupied molecular orbital–lowest occupied molecular orbital (HOMO–LUMO), the Fukui functions, and the Mulliken atomic charge. Overall, the results suggest that HTZS is a good corrosion inhibitor with a large surface area due to the presence of S, N, and O atoms, allowing for creating a larger surface due to the large molecular volume of atoms protecting against the corrosion process.
中文翻译:

由量子力学建模、光谱和表面研究支持的探索性实验分析,在腙基希夫碱存在下对 C 钢表面修复酸性介质中的腐蚀缺陷
这项工作的重点是通过创造负担得起的、可持续的、环保的和高效的耐腐蚀化学品来发展腐蚀控制和保护环境。即通过合成三个腙席夫碱E -2-(1-亚肼基乙基)噻唑( HTZ )、2-(( E )-((( Z )-1-(噻唑-2-基)亚乙基)亚肼基)甲基) 苯酚 ( HTZS ) 和 2-(( E )-((( Z )-1-(吡啶-2-基)亚乙基)亚肼基)甲基)苯酚 ( HPYS ) 和 8 MH 3碳钢缓蚀剂研究的PO 4溶液。使用1 H NMR 和13 C NMR 光谱对这些化学物质进行了分析,以了解更多信息。主要是,基于腙的席夫碱被认为是强大的抑制剂,因为它们能够通过其反应位点(N、O 和 S)以非常低的浓度被吸附。HTZS浓度为 99.00 × 10 –3 mol/L,温度为 293 K 时,可充分发现最大表面 (θ max ) 覆盖率和 83.33% 的抑制效率。恒电流实验表明,提高腙浓度可改善传质阻力。为了研究微观结构,使用了扫描、反射和能量色散 X 射线。通过原子力显微镜评估吸附层的粗糙度和定性粘附力。添加99.00×10-3mol/L的HTZS后,表面亮度和反射率增加到137.20,相对于腐蚀的无电解质溶液的27.70。添加HTZS后,粗糙度 (Ra) 从 0.468 μm 降低至 0.088 μm 。表面形貌研究证实,在碳钢溶解槽中添加腙可以大大改善表面的外观和纹理质量。采用原子吸收光谱技术比较不同条件下不存在和存在腙的情况下恒电流分析后溶液中残留的铁离子浓度;发现与未抑制溶液相比,抑制溶液含有较低浓度的铁离子。 DFT理论分析验证了腙通过遵守动力学吸附等温线的成键电子进行物理吸附的观察。它基于检查最高占据分子轨道-最低占据分子轨道 (HOMO-LUMO)、Fukui 函数和 Mulliken 原子电荷。总体而言,结果表明HTZS是一种良好的腐蚀抑制剂,由于存在 S、N 和 O 原子,因此具有较大的表面积,由于原子分子体积较大,可以形成更大的表面,从而防止腐蚀过程。
更新日期:2024-03-28
中文翻译:

由量子力学建模、光谱和表面研究支持的探索性实验分析,在腙基希夫碱存在下对 C 钢表面修复酸性介质中的腐蚀缺陷
这项工作的重点是通过创造负担得起的、可持续的、环保的和高效的耐腐蚀化学品来发展腐蚀控制和保护环境。即通过合成三个腙席夫碱E -2-(1-亚肼基乙基)噻唑( HTZ )、2-(( E )-((( Z )-1-(噻唑-2-基)亚乙基)亚肼基)甲基) 苯酚 ( HTZS ) 和 2-(( E )-((( Z )-1-(吡啶-2-基)亚乙基)亚肼基)甲基)苯酚 ( HPYS ) 和 8 MH 3碳钢缓蚀剂研究的PO 4溶液。使用1 H NMR 和13 C NMR 光谱对这些化学物质进行了分析,以了解更多信息。主要是,基于腙的席夫碱被认为是强大的抑制剂,因为它们能够通过其反应位点(N、O 和 S)以非常低的浓度被吸附。HTZS浓度为 99.00 × 10 –3 mol/L,温度为 293 K 时,可充分发现最大表面 (θ max ) 覆盖率和 83.33% 的抑制效率。恒电流实验表明,提高腙浓度可改善传质阻力。为了研究微观结构,使用了扫描、反射和能量色散 X 射线。通过原子力显微镜评估吸附层的粗糙度和定性粘附力。添加99.00×10-3mol/L的HTZS后,表面亮度和反射率增加到137.20,相对于腐蚀的无电解质溶液的27.70。添加HTZS后,粗糙度 (Ra) 从 0.468 μm 降低至 0.088 μm 。表面形貌研究证实,在碳钢溶解槽中添加腙可以大大改善表面的外观和纹理质量。采用原子吸收光谱技术比较不同条件下不存在和存在腙的情况下恒电流分析后溶液中残留的铁离子浓度;发现与未抑制溶液相比,抑制溶液含有较低浓度的铁离子。 DFT理论分析验证了腙通过遵守动力学吸附等温线的成键电子进行物理吸附的观察。它基于检查最高占据分子轨道-最低占据分子轨道 (HOMO-LUMO)、Fukui 函数和 Mulliken 原子电荷。总体而言,结果表明HTZS是一种良好的腐蚀抑制剂,由于存在 S、N 和 O 原子,因此具有较大的表面积,由于原子分子体积较大,可以形成更大的表面,从而防止腐蚀过程。















































 京公网安备 11010802027423号
京公网安备 11010802027423号