当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Theoretical Investigation of the Activity of Cobalt Oxides for the Electrochemical Oxidation of Water
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2013-08-30 , DOI: 10.1021/ja405997s Michal Bajdich 1, 2 , Mónica García-Mota 3 , Aleksandra Vojvodic 3 , Jens K. Nørskov 3 , Alexis T. Bell 1, 2
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2013-08-30 , DOI: 10.1021/ja405997s Michal Bajdich 1, 2 , Mónica García-Mota 3 , Aleksandra Vojvodic 3 , Jens K. Nørskov 3 , Alexis T. Bell 1, 2
Affiliation
|
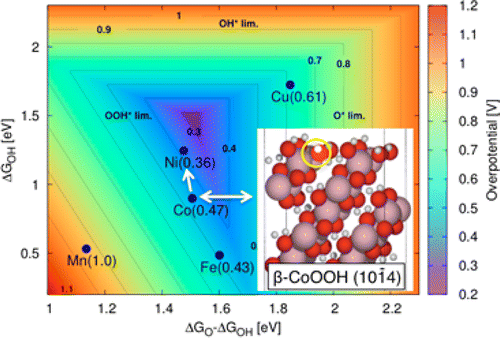
The presence of layered cobalt oxides has been identified experimentally in Co-based anodes under oxygen-evolving conditions. In this work, we report the results of theoretical investigations of the relative stability of layered and spinel bulk phases of Co oxides, as well as the stability of selected surfaces as a function of applied potential and pH. We then study the oxygen evolution reaction (OER) on these surfaces and obtain activity trends at experimentally relevant electro-chemical conditions. Our calculated volume Pourbaix diagram shows that β-CoOOH is the active phase where the OER occurs in alkaline media. We calculate relative surface stabilities and adsorbate coverages of the most stable low-index surfaces of β-CoOOH: (0001), (0112), and (1014). We find that at low applied potentials, the (1014) surface is the most stable, while the (0112) surface is the more stable at higher potentials. Next, we compare the theoretical overpotentials for all three surfaces and find that the (1014) surface is the most active one as characterized by an overpotential of η = 0.48 V. The high activity of the (1014) surface can be attributed to the observation that the resting state of Co in the active site is Co(3+) during the OER, whereas Co is in the Co(4+) state in the less active surfaces. Lastly, we demonstrate that the overpotential of the (1014) surface can be lowered further by surface substitution of Co by Ni. This finding could explain the experimentally observed enhancement in the OER activity of Ni(y)Co(1-y)O(x) thin films with increasing Ni content. All energetics in this work were obtained from density functional theory using the Hubbard-U correction.
中文翻译:

钴氧化物对水电化学氧化活性的理论研究
层状氧化钴的存在已经在氧气释放条件下通过实验确定在 Co 基阳极中。在这项工作中,我们报告了 Co 氧化物层状和尖晶石体相的相对稳定性的理论研究结果,以及所选表面的稳定性作为外加电位和 pH 值的函数。然后,我们研究这些表面上的析氧反应 (OER),并在实验相关的电化学条件下获得活性趋势。我们计算的体积普贝图显示 β-CoOOH 是活性相,其中 OER 发生在碱性介质中。我们计算了 β-CoOOH 最稳定的低指数表面的相对表面稳定性和吸附物覆盖率:(0001)、(0112) 和 (1014)。我们发现在低外加电位下,(1014)表面是最稳定的,而 (0112) 表面在较高电位下更稳定。接下来,我们比较所有三个表面的理论过电位,发现(1014)表面是最活跃的,其特征是 η = 0.48 V。(1014)表面的高活性可归因于观察在 OER 期间,Co 在活性位点的静止状态是 Co(3+),而 Co 在活性较低的表面处于 Co(4+) 状态。最后,我们证明了(1014)表面的过电位可以通过用 Ni 表面取代 Co 来进一步降低。这一发现可以解释实验观察到的 Ni(y)Co(1-y)O(x) 薄膜的 OER 活性随着 Ni 含量的增加而增强。这项工作中的所有能量学都是使用 Hubbard-U 校正从密度泛函理论中获得的。
更新日期:2013-08-30
中文翻译:

钴氧化物对水电化学氧化活性的理论研究
层状氧化钴的存在已经在氧气释放条件下通过实验确定在 Co 基阳极中。在这项工作中,我们报告了 Co 氧化物层状和尖晶石体相的相对稳定性的理论研究结果,以及所选表面的稳定性作为外加电位和 pH 值的函数。然后,我们研究这些表面上的析氧反应 (OER),并在实验相关的电化学条件下获得活性趋势。我们计算的体积普贝图显示 β-CoOOH 是活性相,其中 OER 发生在碱性介质中。我们计算了 β-CoOOH 最稳定的低指数表面的相对表面稳定性和吸附物覆盖率:(0001)、(0112) 和 (1014)。我们发现在低外加电位下,(1014)表面是最稳定的,而 (0112) 表面在较高电位下更稳定。接下来,我们比较所有三个表面的理论过电位,发现(1014)表面是最活跃的,其特征是 η = 0.48 V。(1014)表面的高活性可归因于观察在 OER 期间,Co 在活性位点的静止状态是 Co(3+),而 Co 在活性较低的表面处于 Co(4+) 状态。最后,我们证明了(1014)表面的过电位可以通过用 Ni 表面取代 Co 来进一步降低。这一发现可以解释实验观察到的 Ni(y)Co(1-y)O(x) 薄膜的 OER 活性随着 Ni 含量的增加而增强。这项工作中的所有能量学都是使用 Hubbard-U 校正从密度泛函理论中获得的。

































 京公网安备 11010802027423号
京公网安备 11010802027423号