当前位置:
X-MOL 学术
›
Biochem. Eng. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Study on one-step purification and immobilization of glycosyltransferase by modified Fe3O4 for the synthesis of rare ginsenoside Rh2
Biochemical Engineering Journal ( IF 3.7 ) Pub Date : 2024-03-26 , DOI: 10.1016/j.bej.2024.109297
Junsong Yue , Yufei Zhang , Jianwen Wang , Zhiyan Li , Hongyang Long , Xiaochen Liu , Zhansheng Wu
Biochemical Engineering Journal ( IF 3.7 ) Pub Date : 2024-03-26 , DOI: 10.1016/j.bej.2024.109297
Junsong Yue , Yufei Zhang , Jianwen Wang , Zhiyan Li , Hongyang Long , Xiaochen Liu , Zhansheng Wu
|
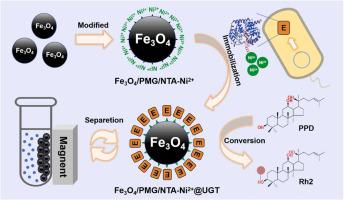
Enzymatic catalysis is the most suitable method for synthesizing the ginsenoside Rh2. However, challenges in the purification and reuse of the enzyme limited the applications of this method. In this study, FeO/PMG/NTA-Ni magnetic nanospheres modified with nano-FeO were employed to achieve the one-step purification and immobilization of glycosyltransferase. The resulting assembly catalyzed ginsenoside protopanaxadiol (PPD) to more valuable Rh2. This material successfully immobilized the recombinant enzyme on the carrier. Moreover, the good dispersibility and superparamagnetism of the immobilized enzyme were maintained without any alteration in its structure, and the maximum immobilized enzyme amount was 180 mg/g. Notably, the pH value, temperature and storage stability of the immobilized enzyme were significantly improved compared with the free enzyme. After reusing the immobilized enzyme six times, the relative enzyme activity was still maintained at 67.8%. Thus, this study specifically adsorbed, purified, and immobilized free glycosyltransferase by modifying nanometer-sized FeO particles. This reduced operating costs and enhanced the properties of the enzyme.
中文翻译:

修饰Fe3O4一步纯化固定糖基转移酶用于合成稀有人参皂苷Rh2的研究
酶催化是合成人参皂苷Rh2最合适的方法。然而,酶的纯化和再利用方面的挑战限制了该方法的应用。本研究采用纳米Fe3O修饰的Fe3O/PMG/NTA-Ni磁性纳米球实现了糖基转移酶的一步纯化和固定化。由此产生的组装体催化人参皂苷原人参二醇 (PPD) 生成更有价值的 Rh2。该材料成功地将重组酶固定在载体上。而且固定化酶结构未发生任何改变,保持了良好的分散性和超顺磁性,最大固定化酶量为180 mg/g。值得注意的是,与游离酶相比,固定化酶的pH值、温度和储存稳定性均显着提高。固定化酶重复使用6次后,相对酶活力仍保持在67.8%。因此,本研究通过修饰纳米尺寸的Fe3O颗粒来特异性吸附、纯化和固定游离糖基转移酶。这降低了运营成本并增强了酶的特性。
更新日期:2024-03-26
中文翻译:

修饰Fe3O4一步纯化固定糖基转移酶用于合成稀有人参皂苷Rh2的研究
酶催化是合成人参皂苷Rh2最合适的方法。然而,酶的纯化和再利用方面的挑战限制了该方法的应用。本研究采用纳米Fe3O修饰的Fe3O/PMG/NTA-Ni磁性纳米球实现了糖基转移酶的一步纯化和固定化。由此产生的组装体催化人参皂苷原人参二醇 (PPD) 生成更有价值的 Rh2。该材料成功地将重组酶固定在载体上。而且固定化酶结构未发生任何改变,保持了良好的分散性和超顺磁性,最大固定化酶量为180 mg/g。值得注意的是,与游离酶相比,固定化酶的pH值、温度和储存稳定性均显着提高。固定化酶重复使用6次后,相对酶活力仍保持在67.8%。因此,本研究通过修饰纳米尺寸的Fe3O颗粒来特异性吸附、纯化和固定游离糖基转移酶。这降低了运营成本并增强了酶的特性。

































 京公网安备 11010802027423号
京公网安备 11010802027423号