当前位置:
X-MOL 学术
›
Bioorgan. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Design, synthesis and biological evaluation of new 2,6-difluorinated phenyl 4-(2-oxoimidazolidin-1-yl)benzenesulfonates as new antimicrotubule agents
Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2024-03-20 , DOI: 10.1016/j.bioorg.2024.107299 Chahrazed Bouzriba , Mathieu Gagné-Boulet , Atziri Corin Chavez Alvarez , Vincent Ouellette , Isabelle Laverdière , Sébastien Fortin
Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2024-03-20 , DOI: 10.1016/j.bioorg.2024.107299 Chahrazed Bouzriba , Mathieu Gagné-Boulet , Atziri Corin Chavez Alvarez , Vincent Ouellette , Isabelle Laverdière , Sébastien Fortin
|
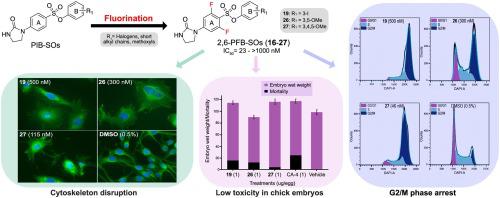
We previously discovered a novel family of antimicrotubule agents designated as phenyl 4-(2-oxoimidazolidin-1-yl)benzenesulfonates (PIB-SOs). In this study, we evaluated the effect of the difluorination of the aromatic ring bearing the imidazolidin-2-one moiety (ring A) at positions 3, 5 and 2, 6 on their antiproliferative activity on four cancer cell lines, their ability to disrupt the microtubules and their toxicity toward chick embryos. We thus synthesized, characterized and biologically evaluated 24 new difluorinated PIB-SO derivatives designated as phenyl 3,5-difluoro-4-(2-oxoimidazolidin-1-yl)benzenesulfonates (3,5-PFB-SOs, –) and phenyl 2,6-difluoro-4-(2-oxoimidazolidin-1-yl)benzenesulfonates (2,6-PFB-SOs, –). The concentration of the drug required to inhibit cell growth by 50% (IC) of 3,5-PFB-SOs is over 1000 nM while most of 2,6-PFB-SOs exhibit IC in the nanomolar range (23–900 nM). Furthermore, the most potent 2,6-PFB-SOs , and arrest the cell cycle progression in G2/M phase, induce cytoskeleton disruption and impair microtubule polymerization. Docking studies also show that the most potent 2,6-PFB-SOs , , , and have binding affinity toward the colchicine-binding site (C-BS). Moreover, their antiproliferative activity is not affected by antimicrotubule- and multidrug-resistant cell lines. Besides, they exhibit improved hepatic stability in the mouse, rat and human microsomes compared to their non-fluorinated counterparts. They also showed theoretical pharmacokinetic, physicochemical and drug-like properties suited for further assays. In addition, they exhibit low to no systemic toxicity toward chick embryos. Finally, our study evidences that PIB-SOs must be fluorinated in specific positions on ring A to maintain both their antiproliferative activity and their biological activity toward microtubules.
中文翻译:

新型抗微管药物2,6-二氟苯基4-(2-氧代咪唑烷-1-基)苯磺酸盐的设计、合成及生物学评价
我们之前发现了一个新的抗微管药物家族,称为 4-(2-氧代咪唑烷-1-基)苯磺酸苯酯 (PIB-SO)。在这项研究中,我们评估了 3、5 和 2、6 位带有咪唑啉-2-酮部分(环 A)的芳环的二氟化对其对四种癌细胞系的抗增殖活性、破坏能力的影响。微管及其对鸡胚胎的毒性。因此,我们合成、表征和生物学评估了 24 种新的二氟化 PIB-SO 衍生物,命名为苯基 3,5-二氟-4-(2-氧代咪唑烷-1-基)苯磺酸盐 (3,5-PFB-SOs, –) 和苯基 2 ,6-二氟-4-(2-氧代咪唑啉-1-基)苯磺酸盐(2,6-PFB-SOs, –)。 3,5-PFB-SO 抑制细胞生长 50% (IC) 所需的药物浓度超过 1000 nM,而大多数 2,6-PFB-SO 的 IC 浓度在纳摩尔范围内 (23–900 nM) 。此外,最有效的 2,6-PFB-SO 可以将细胞周期进程阻滞在 G2/M 期,诱导细胞骨架破坏并损害微管聚合。对接研究还表明,最有效的 2,6-PFB-SO 、 、 、 和 对秋水仙碱结合位点 (C-BS) 具有结合亲和力。此外,它们的抗增殖活性不受抗微管和多重耐药细胞系的影响。此外,与非氟化对应物相比,它们在小鼠、大鼠和人类微粒体中表现出改善的肝脏稳定性。他们还显示了适合进一步分析的理论药代动力学、物理化学和药物样特性。此外,它们对鸡胚的全身毒性很低甚至没有。最后,我们的研究证明,PIB-SO 必须在 A 环上的特定位置进行氟化,才能维持其抗增殖活性和对微管的生物活性。
更新日期:2024-03-20
中文翻译:

新型抗微管药物2,6-二氟苯基4-(2-氧代咪唑烷-1-基)苯磺酸盐的设计、合成及生物学评价
我们之前发现了一个新的抗微管药物家族,称为 4-(2-氧代咪唑烷-1-基)苯磺酸苯酯 (PIB-SO)。在这项研究中,我们评估了 3、5 和 2、6 位带有咪唑啉-2-酮部分(环 A)的芳环的二氟化对其对四种癌细胞系的抗增殖活性、破坏能力的影响。微管及其对鸡胚胎的毒性。因此,我们合成、表征和生物学评估了 24 种新的二氟化 PIB-SO 衍生物,命名为苯基 3,5-二氟-4-(2-氧代咪唑烷-1-基)苯磺酸盐 (3,5-PFB-SOs, –) 和苯基 2 ,6-二氟-4-(2-氧代咪唑啉-1-基)苯磺酸盐(2,6-PFB-SOs, –)。 3,5-PFB-SO 抑制细胞生长 50% (IC) 所需的药物浓度超过 1000 nM,而大多数 2,6-PFB-SO 的 IC 浓度在纳摩尔范围内 (23–900 nM) 。此外,最有效的 2,6-PFB-SO 可以将细胞周期进程阻滞在 G2/M 期,诱导细胞骨架破坏并损害微管聚合。对接研究还表明,最有效的 2,6-PFB-SO 、 、 、 和 对秋水仙碱结合位点 (C-BS) 具有结合亲和力。此外,它们的抗增殖活性不受抗微管和多重耐药细胞系的影响。此外,与非氟化对应物相比,它们在小鼠、大鼠和人类微粒体中表现出改善的肝脏稳定性。他们还显示了适合进一步分析的理论药代动力学、物理化学和药物样特性。此外,它们对鸡胚的全身毒性很低甚至没有。最后,我们的研究证明,PIB-SO 必须在 A 环上的特定位置进行氟化,才能维持其抗增殖活性和对微管的生物活性。








































 京公网安备 11010802027423号
京公网安备 11010802027423号