当前位置:
X-MOL 学术
›
Chem. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
High Li-Ion Conductivity in Pyrochlore-Type Solid Electrolyte Li2–xLa(1+x)/3M2O6F (M = Nb, Ta)
Chemistry of Materials ( IF 7.2 ) Pub Date : 2024-03-28 , DOI: 10.1021/acs.chemmater.3c03288 Akihisa Aimi 1 , Hitoshi Onodera 2 , Yuta Shimonishi 2 , Kenjiro Fujimoto 1 , Shuhei Yoshida 3
Chemistry of Materials ( IF 7.2 ) Pub Date : 2024-03-28 , DOI: 10.1021/acs.chemmater.3c03288 Akihisa Aimi 1 , Hitoshi Onodera 2 , Yuta Shimonishi 2 , Kenjiro Fujimoto 1 , Shuhei Yoshida 3
Affiliation
|
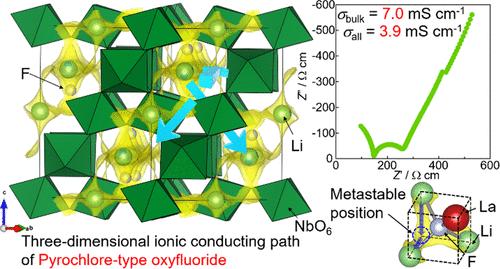
All-solid-state Li-ion batteries featuring both a high energy density and safety are desirable. Sulfide-based solid electrolytes with high conductivities have been actively studied. However, such electrolytes easily react with moisture in air to generate toxic H2S. Therefore, non-sulfide-based solid electrolytes with high ionic conductivity are needed. In this study, we discovered high ionic conductivity in pyrochlore-type oxyfluoride Li2–xLa(1+x)/3M2O6F (M = Nb, Ta), which was stable in air. Li1.25La0.58Nb2O6F exhibited a bulk ionic conductivity of 7.0 mS cm–1 and a total ionic conductivity of 3.9 mS cm–1 at room temperature (∼298 K), which are higher than those of any previously reported oxide solid electrolytes. The conduction path of pyrochlore-type structure covers the F ions located in the tunnels created by MO6 octahedra. The conduction mechanism is the sequential movement of Li ions while changing bonds with F ions. Li ions move to the nearest Li position always passing through metastable positions. Immobile La3+ bonded to the F ion inhibits the Li-ion conduction by blocking the conduction path and vanishing the surrounding metastable positions. We not only successfully synthesized a Li-ion conductor with high conductivity and stability in air but also pioneered a new class of superionic conductors with a pyrochlore-type oxyfluoride.
中文翻译:

烧绿石型固体电解质 Li2–xLa(1+x)/3M2O6F (M = Nb, Ta) 具有高锂离子电导率
兼具高能量密度和安全性的全固态锂离子电池是人们所期望的。具有高电导率的硫化物基固体电解质已被积极研究。然而,此类电解质容易与空气中的水分反应生成有毒的H 2 S。因此,需要具有高离子电导率的非硫化物基固体电解质。在本研究中,我们发现烧绿石型氟氧化物Li 2– x La (1+ x )/3 M 2 O 6 F (M = Nb, Ta)具有高离子电导率,且在空气中稳定。 Li 1.25 La 0.58 Nb 2 O 6 F在室温(~298 K)下表现出7.0 mS cm –1的体离子电导率和3.9 mS cm –1的总离子电导率,高于任何先前报道的氧化物固体电解质。烧绿石型结构的传导路径覆盖位于MO 6八面体形成的隧道中的F离子。传导机制是Li离子的顺序运动,同时改变与F离子的键。 Li离子总是穿过亚稳态位置移动到最近的Li位置。与 F 离子结合的固定 La 3+通过阻断传导路径并消除周围的亚稳态位置来抑制锂离子传导。我们不仅成功合成了在空气中具有高电导率和稳定性的锂离子导体,而且开创了一种新型的烧绿石型氟氧化物超离子导体。
更新日期:2024-03-28
中文翻译:

烧绿石型固体电解质 Li2–xLa(1+x)/3M2O6F (M = Nb, Ta) 具有高锂离子电导率
兼具高能量密度和安全性的全固态锂离子电池是人们所期望的。具有高电导率的硫化物基固体电解质已被积极研究。然而,此类电解质容易与空气中的水分反应生成有毒的H 2 S。因此,需要具有高离子电导率的非硫化物基固体电解质。在本研究中,我们发现烧绿石型氟氧化物Li 2– x La (1+ x )/3 M 2 O 6 F (M = Nb, Ta)具有高离子电导率,且在空气中稳定。 Li 1.25 La 0.58 Nb 2 O 6 F在室温(~298 K)下表现出7.0 mS cm –1的体离子电导率和3.9 mS cm –1的总离子电导率,高于任何先前报道的氧化物固体电解质。烧绿石型结构的传导路径覆盖位于MO 6八面体形成的隧道中的F离子。传导机制是Li离子的顺序运动,同时改变与F离子的键。 Li离子总是穿过亚稳态位置移动到最近的Li位置。与 F 离子结合的固定 La 3+通过阻断传导路径并消除周围的亚稳态位置来抑制锂离子传导。我们不仅成功合成了在空气中具有高电导率和稳定性的锂离子导体,而且开创了一种新型的烧绿石型氟氧化物超离子导体。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号