当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Mechanistic insights into diversified photoluminescence behaviours of BF2 complexes of N-benzoyl 2-aminobenzothiazoles
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2024-03-28 , DOI: 10.1039/d4cp00101j He Zheng 1 , Yan-Xue Li 1 , Wen-Chao Xiong 1 , Xing-Cong Wang 1 , Shan-Shan Gong 1 , Shouzhi Pu 2 , Rongwei Shi 3 , Qi Sun 1
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2024-03-28 , DOI: 10.1039/d4cp00101j He Zheng 1 , Yan-Xue Li 1 , Wen-Chao Xiong 1 , Xing-Cong Wang 1 , Shan-Shan Gong 1 , Shouzhi Pu 2 , Rongwei Shi 3 , Qi Sun 1
Affiliation
|
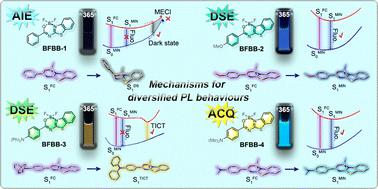
Many BF2 complexes of heteroaromatics are well known for their dual-state emission (DSE) properties. However, AIE and ACQ effects have also been observed in certain cases. To date, no rational explanations have been proposed for these uncommon photoluminescence (PL) behaviours. The current research prepared four BF2 complexes of N-benzoyl 2-aminobenzothiazoles with diversified photoluminescence (PL) properties as model compounds and utilized quantum chemical calculation tools to address this issue. Theoretical calculations revealed that the electron-donating groups (EDGs) at the para-position of the exocyclic phenyl ring exert significant influence on their ground-state electronic structures and vertical excitation features. Potential energy curve (PEC) analysis showed that the exocyclic phenyl ring and NMe2 could not function as effective rotors due to elevated energy barriers. Only the NPh2 of BFBB-3 could spontaneously rotate ∼60° to induce the formation of an emissive twisted intramolecular charge transfer (TICT) state. The two-channel model involving both vibronic relaxation and S0/S1 surface crossing revealed that the drastic narrowing of the S1/S0 energy gap in the region approaching minimun energy conical intersection (MECI) led to the generation of a dark state in BFBB-1. The small energy barrier to access the dark-state region makes the resulting fast internal conversion a competitive channel for excited-state deactivation. In contrast, the presence of EDGs in BFBB-2 and 4 inhibits this pathway, thereby resulting in intense fluorescence emissions in solution. In addition, crystallographic analysis illustrated that the F atoms perpendicular to the polyheterocycle promoted a slipped face-to-face packing mode and enhanced intermolecular interactions. The efficiencies of their solid-state emissions are mainly affected by the degree of π–π overlaps.
中文翻译:

N-苯甲酰基2-氨基苯并噻唑的BF2配合物多样化光致发光行为的机理研究
许多杂芳烃的 BF 2配合物因其双态发射 (DSE) 特性而闻名。然而,在某些情况下也观察到了 AIE 和 ACQ 效应。迄今为止,尚未对这些不常见的光致发光(PL)行为提出合理的解释。目前的研究制备了四种具有多种光致发光(PL)特性的N-苯甲酰基2-氨基苯并噻唑的BF 2配合物作为模型化合物,并利用量子化学计算工具来解决这一问题。理论计算表明,环外苯环对位的给电子基团(EDG)对其基态电子结构和垂直激发特性具有显着影响。势能曲线(PEC)分析表明,由于能垒升高,环外苯环和NMe 2不能充当有效的转子。只有BFBB-3的NPh 2可以自发旋转~60°以诱导发射扭曲分子内电荷转移(TICT)态的形成。涉及振动弛豫和S 0 /S 1表面交叉的双通道模型表明,接近最小能量圆锥相交(MECI)区域中S 1 /S 0能隙的急剧缩小导致暗态的产生在BFBB-1中。进入暗态区域的小能量势垒使得由此产生的快速内部转换成为激发态失活的竞争通道。相反,BFBB-2和4中 EDG 的存在会抑制该途径,从而导致溶液中产生强烈的荧光发射。此外,晶体学分析表明,垂直于多杂环的F原子促进了滑动的面对面堆积模式并增强了分子间相互作用。其固态发射效率主要受π-π重叠程度的影响。
更新日期:2024-03-28
中文翻译:

N-苯甲酰基2-氨基苯并噻唑的BF2配合物多样化光致发光行为的机理研究
许多杂芳烃的 BF 2配合物因其双态发射 (DSE) 特性而闻名。然而,在某些情况下也观察到了 AIE 和 ACQ 效应。迄今为止,尚未对这些不常见的光致发光(PL)行为提出合理的解释。目前的研究制备了四种具有多种光致发光(PL)特性的N-苯甲酰基2-氨基苯并噻唑的BF 2配合物作为模型化合物,并利用量子化学计算工具来解决这一问题。理论计算表明,环外苯环对位的给电子基团(EDG)对其基态电子结构和垂直激发特性具有显着影响。势能曲线(PEC)分析表明,由于能垒升高,环外苯环和NMe 2不能充当有效的转子。只有BFBB-3的NPh 2可以自发旋转~60°以诱导发射扭曲分子内电荷转移(TICT)态的形成。涉及振动弛豫和S 0 /S 1表面交叉的双通道模型表明,接近最小能量圆锥相交(MECI)区域中S 1 /S 0能隙的急剧缩小导致暗态的产生在BFBB-1中。进入暗态区域的小能量势垒使得由此产生的快速内部转换成为激发态失活的竞争通道。相反,BFBB-2和4中 EDG 的存在会抑制该途径,从而导致溶液中产生强烈的荧光发射。此外,晶体学分析表明,垂直于多杂环的F原子促进了滑动的面对面堆积模式并增强了分子间相互作用。其固态发射效率主要受π-π重叠程度的影响。








































 京公网安备 11010802027423号
京公网安备 11010802027423号