当前位置:
X-MOL 学术
›
Eur. J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis and biological activity of thiophene bioisosteres of natural styryl lactone goniofufurone and related compounds
European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2024-03-21 , DOI: 10.1016/j.ejmech.2024.116340 Mirjana Popsavin , Sanja Djokić , Ivana Kovačević , Slađana M. Stanisavljević , Vesna Kojić , Marko V. Rodić , Lidija Aleksić , Jelena Kesić , Bojana Srećo Zelenović , Velimir Popsavin , Dimitar S. Jakimov
European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2024-03-21 , DOI: 10.1016/j.ejmech.2024.116340 Mirjana Popsavin , Sanja Djokić , Ivana Kovačević , Slađana M. Stanisavljević , Vesna Kojić , Marko V. Rodić , Lidija Aleksić , Jelena Kesić , Bojana Srećo Zelenović , Velimir Popsavin , Dimitar S. Jakimov
|
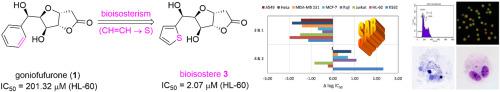
Ten new thiophene derivatives related to goniofufurone have been obtained by multistep synthesis starting from -glucose. The critical step of the synthesis was the Grignard reaction of 2-thienyl magnesium bromide with a protected dialdose, yielding the C-5 epimeric thiophene derivatives and . The mixture was oxidized to the 5-keto derivative , which after deprotection was converted to the corresponding keto-lactone . Stereoselective reduction of afforded the thiophene mimic of goniofufurone . Esterification of with cinnamic or 4-fluorocinnamic acid gave hybrids –. Synthesized analogues were evaluated for their cytotoxicity against several tumour cell lines. The vast majority of them showed better activity than lead . In the culture of K562 cells, compound was more active than the commercial antitumour drug doxorubicin. Structural features of analogues important for their antiproliferative activities were identified by SAR analysis. Pro-apoptotic potential examination of compound on the K562 cell line was performed using flow cytometry, double fluorescence staining and apoptotic morphology screening. Results show that this derivative induces cell membrane disruptions attributable to apoptosis and induces the apoptotic morphology, but decreasing simultaneously the population of cells in the subG1 phase of the cell cycle. The results further suggest that analogue achieves strong cytotoxicity without causing DNA fragmentation. This is clearly indicated by the relatively low incidence of micronuclei, as well as the SAR analysis of all biological effects.
中文翻译:

天然苯乙烯基内酯角异呋喃酮及相关化合物的噻吩生物等排体的合成及生物活性
以β-葡萄糖为原料,通过多步合成得到了十种与goniofufurone相关的新噻吩衍生物。合成的关键步骤是 2-噻吩基溴化镁与受保护的二醛糖发生格氏反应,生成 C-5 差向异构噻吩衍生物和 。混合物被氧化为 5-酮衍生物,脱保护后转化为相应的酮内酯。立体选择性还原得到了goniofufurone的噻吩模拟物。与肉桂酸或4-氟肉桂酸的酯化得到杂化物-。评估了合成类似物对几种肿瘤细胞系的细胞毒性。其中绝大多数表现出比铅更好的活性。在 K562 细胞培养中,该化合物比商业抗肿瘤药物阿霉素更具活性。通过 SAR 分析鉴定了对其抗增殖活性重要的类似物的结构特征。使用流式细胞术、双荧光染色和凋亡形态学筛选进行化合物对K562细胞系的促凋亡潜力检查。结果表明,该衍生物诱导细胞膜因细胞凋亡而破裂,并诱导细胞凋亡形态,但同时减少细胞周期亚G1期的细胞群。结果进一步表明,类似物具有很强的细胞毒性,且不会引起 DNA 断裂。相对较低的微核发生率以及所有生物效应的 SAR 分析清楚地表明了这一点。
更新日期:2024-03-21
中文翻译:

天然苯乙烯基内酯角异呋喃酮及相关化合物的噻吩生物等排体的合成及生物活性
以β-葡萄糖为原料,通过多步合成得到了十种与goniofufurone相关的新噻吩衍生物。合成的关键步骤是 2-噻吩基溴化镁与受保护的二醛糖发生格氏反应,生成 C-5 差向异构噻吩衍生物和 。混合物被氧化为 5-酮衍生物,脱保护后转化为相应的酮内酯。立体选择性还原得到了goniofufurone的噻吩模拟物。与肉桂酸或4-氟肉桂酸的酯化得到杂化物-。评估了合成类似物对几种肿瘤细胞系的细胞毒性。其中绝大多数表现出比铅更好的活性。在 K562 细胞培养中,该化合物比商业抗肿瘤药物阿霉素更具活性。通过 SAR 分析鉴定了对其抗增殖活性重要的类似物的结构特征。使用流式细胞术、双荧光染色和凋亡形态学筛选进行化合物对K562细胞系的促凋亡潜力检查。结果表明,该衍生物诱导细胞膜因细胞凋亡而破裂,并诱导细胞凋亡形态,但同时减少细胞周期亚G1期的细胞群。结果进一步表明,类似物具有很强的细胞毒性,且不会引起 DNA 断裂。相对较低的微核发生率以及所有生物效应的 SAR 分析清楚地表明了这一点。








































 京公网安备 11010802027423号
京公网安备 11010802027423号