当前位置:
X-MOL 学术
›
Eur. J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Guanidine-to-piperidine switch affords high affinity small molecule NPFF ligands with preference for NPFF1-R and NPFF2-R subtypes
European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2024-03-23 , DOI: 10.1016/j.ejmech.2024.116330 Kareem A Galal 1 , Samuel Obeng 2 , Victoria L C Pallares 1 , Alexandria Senetra 3 , Maria A B L Seabra 1 , Ahmed Awad 1 , Christopher R McCurdy 4
European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2024-03-23 , DOI: 10.1016/j.ejmech.2024.116330 Kareem A Galal 1 , Samuel Obeng 2 , Victoria L C Pallares 1 , Alexandria Senetra 3 , Maria A B L Seabra 1 , Ahmed Awad 1 , Christopher R McCurdy 4
Affiliation
|
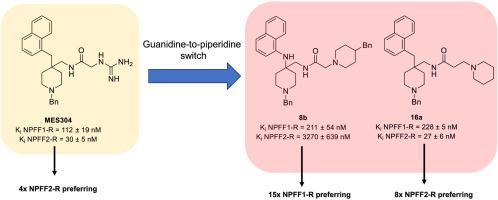
The Neuropeptide FF (NPFF) receptor system is known to modulate opioid actions and has been shown to mediate opioid-induced hyperalgesia and tolerance. The lack of subtype selective small molecule compounds has hampered further exploration of the pharmacology of this receptor system. The vast majority of available NPFF ligands possess a highly basic guanidine group, including our lead small molecule, . Despite providing strong receptor binding, the guanidine group presents a potential pharmacokinetic liability for pharmacological tool development. Through structure-activity relationship exploration, we were able to modify our lead molecule to arrive at guanidine-free NPFF ligands. The novel piperidine analogues and are among the few non-guanidine based NPFF ligands known in literature. Both compounds displayed nanomolar NPFF-R binding affinity approaching that of the parent molecule. Moreover, while was non-subtype selective, these two analogues presented new starting points for subtype selective scaffolds, whereby displayed a 15-fold preference for NPFF1-R, and demonstrated an 8-fold preference for NPFF2-R. Both analogues showed no agonist activity on either receptor subtype in the functional activity assay, while displayed antagonistic properties at NPFF1-R. The calculated physicochemical properties of and were also shown to be more favorable for tool design. These results indicate the possibility of developing potent, subtype selective NPFF ligands devoid of a guanidine functionality.
中文翻译:

胍-哌啶开关提供高亲和力小分子 NPFF 配体,优先选择 NPFF1-R 和 NPFF2-R 亚型
已知神经肽 FF (NPFF) 受体系统可调节阿片类药物的作用,并已被证明可介导阿片类药物引起的痛觉过敏和耐受。亚型选择性小分子化合物的缺乏阻碍了对该受体系统药理学的进一步探索。绝大多数可用的 NPFF 配体都具有高碱性胍基,包括我们的领先小分子 。尽管提供了强大的受体结合作用,胍基团仍为药理学工具开发提供了潜在的药代动力学倾向。通过构效关系探索,我们能够修饰我们的先导分子以获得不含胍的 NPFF 配体。新型哌啶类似物是文献中已知的少数非胍基 NPFF 配体之一。两种化合物均表现出接近母体分子的纳摩尔 NPFF-R 结合亲和力。此外,虽然是非亚型选择性的,但这两种类似物为亚型选择性支架提供了新的起点,从而对 NPFF1-R 显示出 15 倍的偏好,并对 NPFF2-R 显示出 8 倍的偏好。在功能活性测定中,两种类似物对任一受体亚型均未表现出激动剂活性,而对 NPFF1-R 表现出拮抗特性。计算出的物理化学性质 和 也被证明更有利于工具设计。这些结果表明开发有效的、亚型选择性的、不含胍官能团的 NPFF 配体的可能性。
更新日期:2024-03-23
中文翻译:

胍-哌啶开关提供高亲和力小分子 NPFF 配体,优先选择 NPFF1-R 和 NPFF2-R 亚型
已知神经肽 FF (NPFF) 受体系统可调节阿片类药物的作用,并已被证明可介导阿片类药物引起的痛觉过敏和耐受。亚型选择性小分子化合物的缺乏阻碍了对该受体系统药理学的进一步探索。绝大多数可用的 NPFF 配体都具有高碱性胍基,包括我们的领先小分子 。尽管提供了强大的受体结合作用,胍基团仍为药理学工具开发提供了潜在的药代动力学倾向。通过构效关系探索,我们能够修饰我们的先导分子以获得不含胍的 NPFF 配体。新型哌啶类似物是文献中已知的少数非胍基 NPFF 配体之一。两种化合物均表现出接近母体分子的纳摩尔 NPFF-R 结合亲和力。此外,虽然是非亚型选择性的,但这两种类似物为亚型选择性支架提供了新的起点,从而对 NPFF1-R 显示出 15 倍的偏好,并对 NPFF2-R 显示出 8 倍的偏好。在功能活性测定中,两种类似物对任一受体亚型均未表现出激动剂活性,而对 NPFF1-R 表现出拮抗特性。计算出的物理化学性质 和 也被证明更有利于工具设计。这些结果表明开发有效的、亚型选择性的、不含胍官能团的 NPFF 配体的可能性。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号