当前位置:
X-MOL 学术
›
Adv. Funct. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Unveiling the Energy Storage Mechanism of MnO2 Polymorphs for Zinc-Manganese Dioxide Batteries
Advanced Functional Materials ( IF 18.5 ) Pub Date : 2024-03-27 , DOI: 10.1002/adfm.202306652
Qingyong Zhang 1 , Jun Zhao 1 , Xiuyuan Chen 1 , Ruijie Yang 1 , Ting Ying 1 , Chong Cheng 2 , Bilu Liu 3 , Jun Fan 1 , Shuang Li 2 , Zhiyuan Zeng 1, 4
Advanced Functional Materials ( IF 18.5 ) Pub Date : 2024-03-27 , DOI: 10.1002/adfm.202306652
Qingyong Zhang 1 , Jun Zhao 1 , Xiuyuan Chen 1 , Ruijie Yang 1 , Ting Ying 1 , Chong Cheng 2 , Bilu Liu 3 , Jun Fan 1 , Shuang Li 2 , Zhiyuan Zeng 1, 4
Affiliation
|
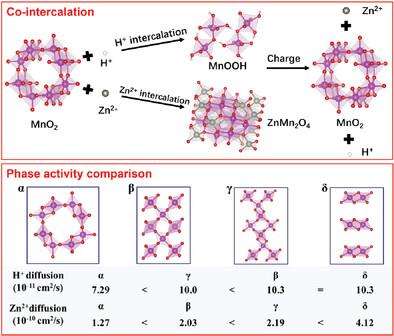
The energy storage mechanism of MnO2 in aqueous zinc ion batteries (ZIBs) is investigated using four types of MnO2 with crystal phases corresponding to α-, β-, γ-, and δ-MnO2. Experimental and theoretical calculation results reveal that all MnO2 follow the H+ and Zn2+ co-intercalation mechanism during discharge, with ZnMn2O4, MnOOH, and Zn4(SO4)(OH)6·4H2O being the main products. ZnMn2O4 is formed from Zn2+ intercalation, while MnOOH and Zn4(SO4)(OH)6·4H2O are formed from H+ intercalation. Charging generates MnO2 through the extraction of Zn2+ and H+ from ZnMn2O4 and MnOOH, indicating reversible reactions. The study also reveals that H+ intercalation exhibits better kinetics than Zn2+ intercalation due to its higher diffusion ability and easier charge transfer. The crystal structures and diffusion energy barriers differences are responsible for the differences of H+ and Zn2+ diffusion in MnO2, with the layered δ phase exhibiting the lowest diffusion energy barrier and highest apparent diffusion coefficient, while the α phase exhibits the highest diffusion energy barrier and lowest apparent diffusion coefficient. These findings highlight the importance of crystal phases in determining the diffusion ability and energy storage of MnO2, which can inform strategies for design of multiphase MnO2 cathodes for high-performance ZIBs.
中文翻译:

揭示二氧化锰电池中 MnO2 多晶型物的储能机制
使用四种类型的 MnO 2 晶相分别对应于 α-、β-、γ- 和δ-MnO 2 。实验和理论计算结果表明,放电过程中所有MnO 2 均遵循H + 和Zn 2+ 共插层机制,其中ZnMn 2 O 4 、MnOOH 和 Zn 4 (SO 4 )(OH) 6 ·4H 2 O为主要产品。 ZnMn 2 O 4 由 Zn 2+ 插层形成,而 MnOOH 和 Zn 4 (SO 4 ) (OH) 6 ·4H 2 O由H + 插层形成。充电时,ZnMn 2 O 4 和 MnOOH 萃取 Zn 2+ 和 H + ,生成 MnO 2 ,表明反应是可逆的。研究还表明,H + 插层由于其更高的扩散能力和更容易的电荷转移而表现出比 Zn 2+ 插层更好的动力学。晶体结构和扩散能垒的差异导致了MnO 2 中H + 和Zn 2+ 扩散的差异,其中层状δ相表现出最低扩散能垒和表观扩散系数最高,而α相的扩散能垒最高,表观扩散系数最低。这些发现强调了晶相在确定 MnO 2 的扩散能力和能量存储方面的重要性,这可以为高性能 ZIB 的多相 MnO 2 阴极的设计策略提供参考。
更新日期:2024-03-27
中文翻译:

揭示二氧化锰电池中 MnO2 多晶型物的储能机制
使用四种类型的 MnO 2 晶相分别对应于 α-、β-、γ- 和δ-MnO 2 。实验和理论计算结果表明,放电过程中所有MnO 2 均遵循H + 和Zn 2+ 共插层机制,其中ZnMn 2 O 4 、MnOOH 和 Zn 4 (SO 4 )(OH) 6 ·4H 2 O为主要产品。 ZnMn 2 O 4 由 Zn 2+ 插层形成,而 MnOOH 和 Zn 4 (SO 4 ) (OH) 6 ·4H 2 O由H + 插层形成。充电时,ZnMn 2 O 4 和 MnOOH 萃取 Zn 2+ 和 H + ,生成 MnO 2 ,表明反应是可逆的。研究还表明,H + 插层由于其更高的扩散能力和更容易的电荷转移而表现出比 Zn 2+ 插层更好的动力学。晶体结构和扩散能垒的差异导致了MnO 2 中H + 和Zn 2+ 扩散的差异,其中层状δ相表现出最低扩散能垒和表观扩散系数最高,而α相的扩散能垒最高,表观扩散系数最低。这些发现强调了晶相在确定 MnO 2 的扩散能力和能量存储方面的重要性,这可以为高性能 ZIB 的多相 MnO 2 阴极的设计策略提供参考。

































 京公网安备 11010802027423号
京公网安备 11010802027423号