当前位置:
X-MOL 学术
›
Mol. Microbiol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Borrelia burgdorferi PlzA is a cyclic-di-GMP dependent DNA and RNA binding protein
Molecular Microbiology ( IF 2.6 ) Pub Date : 2024-03-25 , DOI: 10.1111/mmi.15254 Nerina Jusufovic 1 , Andrew C. Krusenstjerna 1 , Christina R. Savage 1 , Timothy C. Saylor 1 , Catherine A. Brissette 2 , Wolfram R. Zückert 3 , Paula J. Schlax 4 , Md A. Motaleb 5 , Brian Stevenson 1, 6
Molecular Microbiology ( IF 2.6 ) Pub Date : 2024-03-25 , DOI: 10.1111/mmi.15254 Nerina Jusufovic 1 , Andrew C. Krusenstjerna 1 , Christina R. Savage 1 , Timothy C. Saylor 1 , Catherine A. Brissette 2 , Wolfram R. Zückert 3 , Paula J. Schlax 4 , Md A. Motaleb 5 , Brian Stevenson 1, 6
Affiliation
|
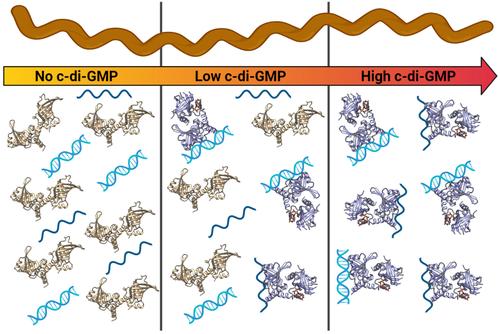
The PilZ domain-containing protein, PlzA, is the only known cyclic di-GMP binding protein encoded by all Lyme disease spirochetes. PlzA has been implicated in the regulation of many borrelial processes, but the effector mechanism of PlzA was not previously known. Here, we report that PlzA can bind DNA and RNA and that nucleic acid binding requires c-di-GMP, with the affinity of PlzA for nucleic acids increasing as concentrations of c-di-GMP were increased. A mutant PlzA that is incapable of binding c-di-GMP did not bind to any tested nucleic acids. We also determined that PlzA interacts predominantly with the major groove of DNA and that sequence length and G–C content play a role in DNA binding affinity. PlzA is a dual-domain protein with a PilZ-like N-terminal domain linked to a canonical C-terminal PilZ domain. Dissection of the domains demonstrated that the separated N-terminal domain bound nucleic acids independently of c-di-GMP. The C-terminal domain, which includes the c-di-GMP binding motifs, did not bind nucleic acids under any tested conditions. Our data are supported by computational docking, which predicts that c-di-GMP binding at the C-terminal domain stabilizes the overall protein structure and facilitates PlzA-DNA interactions via residues in the N-terminal domain. Based on our data, we propose that levels of c-di-GMP during the various stages of the enzootic life cycle direct PlzA binding to regulatory targets.
中文翻译:

伯氏疏螺旋体 PlzA 是一种环二 GMP 依赖性 DNA 和 RNA 结合蛋白
含有 PilZ 结构域的蛋白 PlzA 是唯一已知的由所有莱姆病螺旋体编码的环状二 GMP 结合蛋白。 PlzA 参与了许多疏螺旋体过程的调节,但之前并不知道 PlzA 的效应机制。在这里,我们报道PlzA可以结合DNA和RNA,并且核酸结合需要c-di-GMP,随着c-di-GMP浓度的增加,PlzA对核酸的亲和力增加。不能结合c-di-GMP的突变体PlzA不结合任何测试的核酸。我们还确定 PlzA 主要与 DNA 大沟相互作用,并且序列长度和 G-C 含量在 DNA 结合亲和力中发挥作用。 PlzA 是一种双结构域蛋白,具有与典型 C 端 PilZ 结构域相连的 PilZ 样 N 端结构域。结构域的解剖表明,分离的 N 末端结构域独立于 c-di-GMP 结合核酸。 C 末端结构域包含 c-di-GMP 结合基序,在任何测试条件下均不结合核酸。我们的数据得到了计算对接的支持,计算对接预测 c-di-GMP 在 C 端结构域的结合可以稳定整体蛋白质结构,并通过 N 端结构域中的残基促进 PlzA-DNA 相互作用。根据我们的数据,我们提出,在地方性动物生命周期的各个阶段,c-di-GMP 水平直接指导 PlzA 与监管靶点的结合。
更新日期:2024-03-25
中文翻译:

伯氏疏螺旋体 PlzA 是一种环二 GMP 依赖性 DNA 和 RNA 结合蛋白
含有 PilZ 结构域的蛋白 PlzA 是唯一已知的由所有莱姆病螺旋体编码的环状二 GMP 结合蛋白。 PlzA 参与了许多疏螺旋体过程的调节,但之前并不知道 PlzA 的效应机制。在这里,我们报道PlzA可以结合DNA和RNA,并且核酸结合需要c-di-GMP,随着c-di-GMP浓度的增加,PlzA对核酸的亲和力增加。不能结合c-di-GMP的突变体PlzA不结合任何测试的核酸。我们还确定 PlzA 主要与 DNA 大沟相互作用,并且序列长度和 G-C 含量在 DNA 结合亲和力中发挥作用。 PlzA 是一种双结构域蛋白,具有与典型 C 端 PilZ 结构域相连的 PilZ 样 N 端结构域。结构域的解剖表明,分离的 N 末端结构域独立于 c-di-GMP 结合核酸。 C 末端结构域包含 c-di-GMP 结合基序,在任何测试条件下均不结合核酸。我们的数据得到了计算对接的支持,计算对接预测 c-di-GMP 在 C 端结构域的结合可以稳定整体蛋白质结构,并通过 N 端结构域中的残基促进 PlzA-DNA 相互作用。根据我们的数据,我们提出,在地方性动物生命周期的各个阶段,c-di-GMP 水平直接指导 PlzA 与监管靶点的结合。











































 京公网安备 11010802027423号
京公网安备 11010802027423号