当前位置:
X-MOL 学术
›
J. Agric. Food Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Comparing the Chemistry of Malvidin-3-O-glucoside and Malvidin-3,5-O-diglucoside Networks: A Holistic Approach to the Acidic and Basic Paradigms with Implications in Biological Studies
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2024-03-23 , DOI: 10.1021/acs.jafc.4c00552 André Seco 1 , Ana Rita Pereira 2 , Ambrósio Camuenho 1 , Joana Oliveira 2 , Ricardo Dias 2 , Natércia F Brás 2 , Nuno Basílio 1 , A Jorge Parola 1 , João C Lima 1 , Victor de Freitas 2 , Fernando Pina 1
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2024-03-23 , DOI: 10.1021/acs.jafc.4c00552 André Seco 1 , Ana Rita Pereira 2 , Ambrósio Camuenho 1 , Joana Oliveira 2 , Ricardo Dias 2 , Natércia F Brás 2 , Nuno Basílio 1 , A Jorge Parola 1 , João C Lima 1 , Victor de Freitas 2 , Fernando Pina 1
Affiliation
|
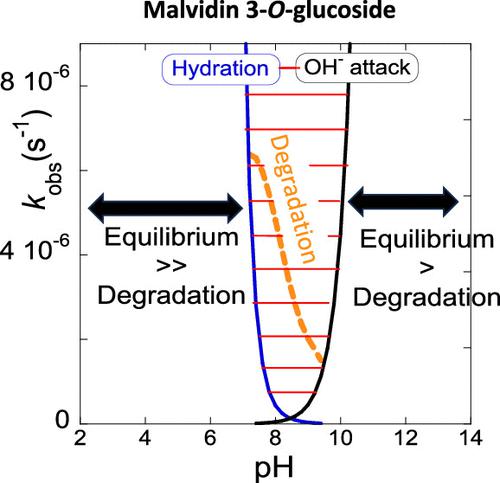
The kinetics, thermodynamics, and degradation of malvidin mono- and diglucosides were studied following a holistic approach by extending to the basic medium. In acidic conditions, the reversible kinetics of the flavylium cation toward the equilibrium is controlled by the hydration and cis–trans isomerization steps, while in the basic medium, the OH– nucleophilic addition to the anionic quinoidal bases is the slowest step. There is a pH range (transition pHs), between the acidic and basic paradigms, that includes physiological pH (7.4), where degradation reactions occur faster, preventing the system from reaching the equilibrium. The transition pH of the diglucoside is narrower, and in contrast with the monoglucoside, there is no evidence for the formation of colored oligomers among the degradation products. Noteworthy, OH– addition in position 4 to form B42–, a kinetic product that decreases the overall equilibration rate, was observed only for the diglucoside.
中文翻译:

比较 Malvidin-3-O-glucoside 和 Malvidin-3,5-O-diglucoside Networks 的化学性质:酸性和碱性范式的整体方法及其在生物学研究中的意义
通过扩展到基本介质,采用整体方法研究了锦葵素单葡萄糖苷和二葡萄糖苷的动力学、热力学和降解。在酸性条件下,黄鎓阳离子向平衡的可逆动力学由水合和顺反异构化步骤控制,而在碱性介质中,阴离子醌基的OH-亲核加成是最慢的步骤。酸性和碱性范例之间存在一个 pH 范围(过渡 pH),其中包括生理 pH (7.4),其中降解反应发生得更快,从而阻止系统达到平衡。二葡萄糖苷的转变pH值较窄,与单葡萄糖苷相比,没有证据表明降解产物中形成有色低聚物。值得注意的是,仅在二葡萄糖苷中观察到在位置 4 上添加 OH -形成 B4 2- ,这是一种降低总体平衡率的动力学产物。
更新日期:2024-03-23
中文翻译:

比较 Malvidin-3-O-glucoside 和 Malvidin-3,5-O-diglucoside Networks 的化学性质:酸性和碱性范式的整体方法及其在生物学研究中的意义
通过扩展到基本介质,采用整体方法研究了锦葵素单葡萄糖苷和二葡萄糖苷的动力学、热力学和降解。在酸性条件下,黄鎓阳离子向平衡的可逆动力学由水合和顺反异构化步骤控制,而在碱性介质中,阴离子醌基的OH-亲核加成是最慢的步骤。酸性和碱性范例之间存在一个 pH 范围(过渡 pH),其中包括生理 pH (7.4),其中降解反应发生得更快,从而阻止系统达到平衡。二葡萄糖苷的转变pH值较窄,与单葡萄糖苷相比,没有证据表明降解产物中形成有色低聚物。值得注意的是,仅在二葡萄糖苷中观察到在位置 4 上添加 OH -形成 B4 2- ,这是一种降低总体平衡率的动力学产物。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号