当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Insight into the Role of TiO2 Facets in Photocatalytic Selective Oxidation of p-Xylene
ACS Catalysis ( IF 11.3 ) Pub Date : 2024-03-26 , DOI: 10.1021/acscatal.4c00543
Xiaowen Sun 1, 2 , Zhendong Feng 2, 3 , Shengyang Wang 2 , Qing-Nan Wang 2 , Pengfei Zhang 2 , Rengui Li 2 , Can Li 2, 3
ACS Catalysis ( IF 11.3 ) Pub Date : 2024-03-26 , DOI: 10.1021/acscatal.4c00543
Xiaowen Sun 1, 2 , Zhendong Feng 2, 3 , Shengyang Wang 2 , Qing-Nan Wang 2 , Pengfei Zhang 2 , Rengui Li 2 , Can Li 2, 3
Affiliation
|
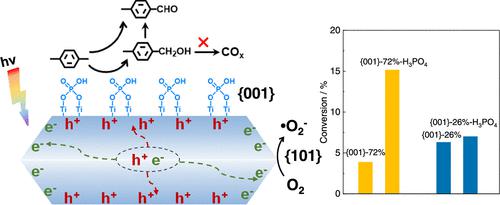
The crystal facets of semiconductors often have critical effects on photocatalytic reactions. The spatial charge separation between different facets in TiO2 reveals a preferential accumulation of photogenerated holes on the {001} facet; thereby, the activation of the C–H bond predominantly occurs on the {001} facet. However, the dissociative adsorption of initially generated p-methylbenzyl alcohol (p-MBY) forms alcoholate species, which impedes the interaction between p-xylene and the {001} facet, and thus blocks the photocatalytic reaction of p-xylene oxidation on {001}-72%. On the other hand, H3PO4 predominantly adsorbs on the {001} facet, with an adsorption energy higher than p-MBY (−5.15 vs −3.90 eV). Therefore, adding H3PO4 can prevent the dissociative adsorption of p-MBY on the {001} facet. The addition of H3PO4 also significantly improves the injection efficiency of photogenerated holes into p-xylene and suppresses the generation of ·O22–, thereby enhancing the conversion and selectivity. Consequently, the addition of H3PO4 obtains a selectivity as high as 94.8% to the primary products at 15.2% conversion on {001}-72%. The results demonstrate that facet engineering for a semiconductor-based photocatalyst can regulate the charge separation, charge injection, and adsorption behavior of intermediates, which is an effective strategy to accomplish high performance of photocatalytic reactions.
中文翻译:

深入了解 TiO2 面在对二甲苯光催化选择性氧化中的作用
半导体的晶面通常对光催化反应具有关键影响。 TiO 2不同晶面之间的空间电荷分离揭示了光生空穴在{001}晶面上的优先积累;因此,C-H键的激活主要发生在{001}面上。然而,最初生成的对甲基苯甲醇(p -MBY)的解离吸附形成醇化物,阻碍了对二甲苯与{001}面之间的相互作用,从而阻碍了对二甲苯氧化在{001上的光催化反应}-72%。另一方面,H 3 PO 4主要吸附在{001}面上,吸附能高于p -MBY(-5.15 vs -3.90 eV)。因此,添加H 3 PO 4可以防止p -MBY在{001}面上的解离吸附。 H 3 PO 4的添加还显着提高了光生空穴向对二甲苯的注入效率,并抑制·O 2 2–的生成,从而提高了转化率和选择性。因此,添加H 3 PO 4可获得高达94.8%的初级产物选择性,{001}-72%的转化率为15.2%。结果表明,半导体光催化剂的面工程可以调节中间体的电荷分离、电荷注入和吸附行为,这是实现高性能光催化反应的有效策略。
更新日期:2024-03-26
中文翻译:

深入了解 TiO2 面在对二甲苯光催化选择性氧化中的作用
半导体的晶面通常对光催化反应具有关键影响。 TiO 2不同晶面之间的空间电荷分离揭示了光生空穴在{001}晶面上的优先积累;因此,C-H键的激活主要发生在{001}面上。然而,最初生成的对甲基苯甲醇(p -MBY)的解离吸附形成醇化物,阻碍了对二甲苯与{001}面之间的相互作用,从而阻碍了对二甲苯氧化在{001上的光催化反应}-72%。另一方面,H 3 PO 4主要吸附在{001}面上,吸附能高于p -MBY(-5.15 vs -3.90 eV)。因此,添加H 3 PO 4可以防止p -MBY在{001}面上的解离吸附。 H 3 PO 4的添加还显着提高了光生空穴向对二甲苯的注入效率,并抑制·O 2 2–的生成,从而提高了转化率和选择性。因此,添加H 3 PO 4可获得高达94.8%的初级产物选择性,{001}-72%的转化率为15.2%。结果表明,半导体光催化剂的面工程可以调节中间体的电荷分离、电荷注入和吸附行为,这是实现高性能光催化反应的有效策略。

































 京公网安备 11010802027423号
京公网安备 11010802027423号