当前位置:
X-MOL 学术
›
J. Agric. Food Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis and Herbicidal Activity of 2-(2-Oxo-3-pyridyl-benzothiazol-6-yloxy)hexanoic Acids
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2024-03-25 , DOI: 10.1021/acs.jafc.3c08213 Yuqian Huang 1 , Zhanbin Li 1 , Yu Chen 1 , Wenqi Li 1 , Shaopeng Wei 1, 2 , Zhiqin Ji 1, 2
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2024-03-25 , DOI: 10.1021/acs.jafc.3c08213 Yuqian Huang 1 , Zhanbin Li 1 , Yu Chen 1 , Wenqi Li 1 , Shaopeng Wei 1, 2 , Zhiqin Ji 1, 2
Affiliation
|
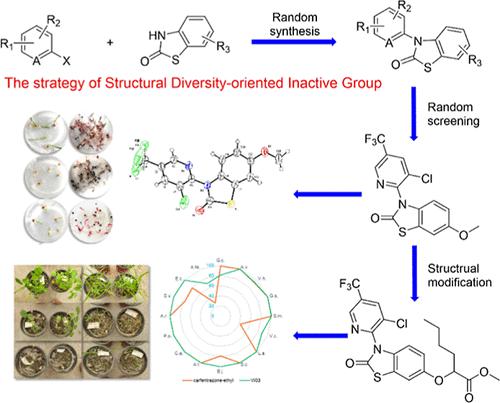
The discovery of a lead compound is fundamental to herbicide innovation, but the limited availability of valuable lead compounds has hindered their development in recent years. By utilizing the structural diversity-oriented inactive group strategy, 3-(2-pyridyl)-benzothiazol-2-one was identified as a promising lead scaffold for herbicides, starting from benzothiazole which is an inactive moiety commonly found in herbicides such as mefenacet, benazolin, benzthiazuron, and fenthiaprop-ethyl. To investigate the structure–activity relationship (SAR) of these chemicals, a series of 2-(2-oxo-3-pyridyl-benzothiazol-6-yloxy)hexanoic acid derivatives (VI01 ∼ VI28) were synthesized through classical nucleophilic SNAr reaction using halogenated pyridines and 6-methoxybenzothiazole-2-one. The chemical structures of all the title compounds were confirmed by NMR and MS analysis. Petri dish assays indicated that many compounds exhibited potent herbicidal activity against both broad-leaf weeds and grass weeds at 1.0 mg/L. The SAR analysis revealed that the presence of a trifluoromethyl group at the 5-position of pyridine is essential for herbicidal activity. Furthermore, carboxylic esters exhibit higher herbicidal activity compared to carboxylic amides and free acids, and the activity decreased with the extension of the carbon chain. The postemergence herbicidal activity of VI03 against 16 species of weeds was tested by pot experiments in a greenhouse. VI03 demonstrated comparable efficacy in controlling broadleaf weeds and superior efficacy in controlling grass weeds compared to carfentrazone ethyl. The present study has unveiled a novel molecular scaffold exhibiting remarkably potent herbicidal activity. These findings are anticipated to provide valuable insights for the advancement of new herbicides and offer an alternative approach for managing resistant weeds.
中文翻译:

2-(2-氧代-3-吡啶基苯并噻唑-6-基氧基)己酸的合成及除草活性
先导化合物的发现是除草剂创新的基础,但有价值的先导化合物的有限供应阻碍了它们近年来的发展。通过利用结构多样性导向的非活性基团策略,3-(2-吡啶基)-苯并噻唑-2-酮被认为是一种有前景的除草剂先导支架,以苯并噻唑为起点,苯并噻唑是除草剂(如苯芬那特)中常见的非活性部分,苯那唑啉、苯并噻唑隆和噻唑啉乙酯。为了研究这些化学物质的构效关系(SAR),通过经典的亲核SN Ar合成了一系列2-(2-氧代-3-吡啶基-苯并噻唑-6-基氧基)己酸衍生物( VI01∼VI28 )使用卤代吡啶和6-甲氧基苯并噻唑-2-酮进行反应。所有标题化合物的化学结构均通过NMR和MS分析证实。培养皿测定表明,许多化合物在 1.0 mg/L 浓度下对阔叶杂草和禾本科杂草均表现出有效的除草活性。 SAR分析表明,吡啶5位上三氟甲基的存在对于除草活性至关重要。此外,与羧酸酰胺和游离酸相比,羧酸酯表现出更高的除草活性,并且活性随着碳链的延长而降低。通过温室盆栽实验测试了VI03对16种杂草的芽后除草活性。与唑草酮相比, VI03在控制阔叶杂草方面表现出相当的功效,在控制禾本科杂草方面表现出更优异的功效。本研究揭示了一种新型分子支架,具有非常有效的除草活性。 这些发现预计将为新型除草剂的进步提供宝贵的见解,并为管理抗性杂草提供替代方法。
更新日期:2024-03-25
中文翻译:

2-(2-氧代-3-吡啶基苯并噻唑-6-基氧基)己酸的合成及除草活性
先导化合物的发现是除草剂创新的基础,但有价值的先导化合物的有限供应阻碍了它们近年来的发展。通过利用结构多样性导向的非活性基团策略,3-(2-吡啶基)-苯并噻唑-2-酮被认为是一种有前景的除草剂先导支架,以苯并噻唑为起点,苯并噻唑是除草剂(如苯芬那特)中常见的非活性部分,苯那唑啉、苯并噻唑隆和噻唑啉乙酯。为了研究这些化学物质的构效关系(SAR),通过经典的亲核SN Ar合成了一系列2-(2-氧代-3-吡啶基-苯并噻唑-6-基氧基)己酸衍生物( VI01∼VI28 )使用卤代吡啶和6-甲氧基苯并噻唑-2-酮进行反应。所有标题化合物的化学结构均通过NMR和MS分析证实。培养皿测定表明,许多化合物在 1.0 mg/L 浓度下对阔叶杂草和禾本科杂草均表现出有效的除草活性。 SAR分析表明,吡啶5位上三氟甲基的存在对于除草活性至关重要。此外,与羧酸酰胺和游离酸相比,羧酸酯表现出更高的除草活性,并且活性随着碳链的延长而降低。通过温室盆栽实验测试了VI03对16种杂草的芽后除草活性。与唑草酮相比, VI03在控制阔叶杂草方面表现出相当的功效,在控制禾本科杂草方面表现出更优异的功效。本研究揭示了一种新型分子支架,具有非常有效的除草活性。 这些发现预计将为新型除草剂的进步提供宝贵的见解,并为管理抗性杂草提供替代方法。






























 京公网安备 11010802027423号
京公网安备 11010802027423号