Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
磁性氧化石墨烯对废水中苯酚和儿茶酚的吸附行为及机理:基于吸附实验、数学模型和分子模拟的综合研究
ACS Omega ( IF 3.7 ) Pub Date : 2024-03-23 , DOI: 10.1021/acsomega.3c09346
Yuhua Wu 1 , Xi Zhang 2 , Caizhu Liu 1 , Lina Tian 1 , Yufan Zhang 1 , Meilin Zhu 2 , Weiye Qiao 3 , Jianbo Wu 1 , Shu Yan 1 , Hui Zhang 1 , Hongcun Bai 1
Affiliation
|
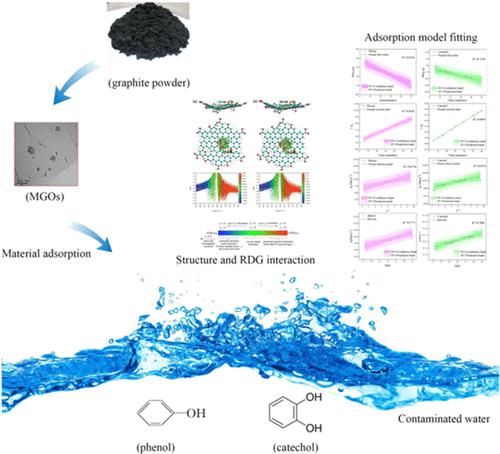
本研究基于吸附实验、数学模型和分子模拟,全面分析了磁性氧化石墨烯(MGO)纳米复合材料对苯酚和儿茶酚的吸附行为和机制。通过系统实验,综合评价了接触时间、pH条件、离子强度等各种参数对吸附效果的影响。吸附的最佳接触时间被确定为 60 分钟,观察到无机盐浓度的增加会对 MGO 对苯酚和儿茶酚的吸附能力产生不利影响。具体而言,MGO在弱酸性条件下表现出优异的吸附性能。朗缪尔模型很好地代表了吸附等温线,表明两种污染物的单层覆盖和有限的吸附位点。就吸附动力学而言,拟一级动力学模型最适合描述苯酚吸附,而儿茶酚吸附更接近拟二级动力学模型,表明这两种相似化合物具有不同的吸附过程。此外,这项研究利用量子化学计算来破译分子水平上的相互作用机制。这种计算提供了相互作用的视觉表示和定量分析,阐明了控制吸附现象的潜在物理和化学力。这些发现不仅可以为含有酚类化合物的煤炭工业废水的处理提供重要的见解,将宏观观察与微观理论解释联系起来,而且还可以促进对水环境中物质与污染物相互作用的理解。

"点击查看英文标题和摘要"




































 京公网安备 11010802027423号
京公网安备 11010802027423号