当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Research and Development on the Manufacturing Process of Dexmedetomidine from Chiral Precursors: Resolution–Racemization–Recycle Synthesis Strategy
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2024-03-20 , DOI: 10.1021/acs.oprd.3c00388 Dmitry Orekhov 1
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2024-03-20 , DOI: 10.1021/acs.oprd.3c00388 Dmitry Orekhov 1
Affiliation
|
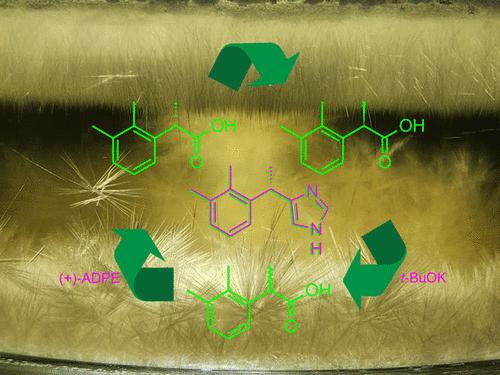
The present work describes the development of a new synthetic route to dexmedetomidine (1), widely used in hospital practice as a versatile anesthetic, free of respiratory distress effects. The conventional approach to the manufacture of compound 1 rests on the resolution of racemic medetomidine (2). However, besides a low recovery of the (S)-enantiomer (∼0.2 kg from one kg of the racemate), the classical resolution suffers from production of chiral waste enriched in levomedetomidine (R-enantiomer), which could not be racemized in a safe and practically applicable manner. We proposed a new precursor for dexmedetomidine, 2-(2,3-dimethylphenyl)propionic acid (5), to be resolved at preliminary steps instead of resolution of dexmedetomidine 1 itself. The resolved acid (S)-enantiomer 4 is then used in the synthesis of 1 and the (R)-enantiomer-enriched chiral waste are readily racemized in a simple, safe, and scalable manner and used again. Further synthesis steps involving (S)-acid 4 included the preparation of sulfur ylide 19 from acid chloride 16 and dimethylsulfoxonium methylide 18, transformation of compound 19 into α-bromoketone 21, and its subsequent transformation to salts 25a–25j. The “azide route” was found to be the optimum one for the preparation of aminoketone, providing the product with acceptable enantiomeric purity, and included the reaction of bromoketone 21 with sodium azide, followed by hydrogenation of the resulting azidoketone 30. The Marckwald cyclization of aminoketone salts 25 with potassium thiocyanate afforded a dexmedetomidine thio derivative 26, the desulfurization of which gave the (S)-enantiomer-enriched target product 1 in high yield. Excellent yields of the racemic acid 5 recycled after resolution and a high percentage recovery of the commercially available chiral amine 11 used for resolution, as well as preliminary enrichment of the crude product with the (S)-enantiomer, allowed us to decrease the amount of chiral waste almost 4-fold compared to the classical resolution of racemic medetomidine (2). In addition, the exclusion of expensive Rh catalysts and chiral ligands, which are used in current methods, considerably decreased the manufacturing cost of 1.
中文翻译:

手性前体制备右美托咪定工艺研究进展:拆分-外消旋化-循环合成策略
目前的工作描述了右美托咪定 ( 1 )的新合成路线的开发,该路线作为一种多功能麻醉剂广泛应用于医院实践,且无呼吸窘迫效应。制备化合物1 的常规方法依赖于外消旋美托咪定 ( 2 ) 的拆分。然而,除了 ( S )-对映异构体的回收率较低(从 1 kg 外消旋体中回收约 0.2 kg)之外,经典拆分还受到产生富含左旋美托咪定(R -对映异构体)的手性废物的影响,该废物无法在安全且实用的方式。我们提出了右美托咪定的新前体 2-(2,3-二甲基苯基)丙酸 ( 5 ),可在初步步骤中进行拆分,而不是拆分右美托咪定1本身。然后将解析的酸 ( S )-对映体4用于合成1,并且富含 ( R )-对映体的手性废物很容易以简单、安全和可扩展的方式外消旋并再次使用。涉及( S )-酸4的进一步合成步骤包括由酰基氯16和二甲基亚砜18制备硫叶立德19 ,将化合物19转化为α-溴酮21,以及随后将其转化为盐25a - 25j。 “叠氮路线”被发现是制备氨基酮的最佳路线,提供了具有可接受的对映体纯度的产物,并且包括溴酮21与叠氮化钠反应,然后氢化所得叠氮酮30。氨基酮盐25与硫氰酸钾的马克瓦尔德环化得到右美托咪定硫代衍生物26,其脱硫以高收率得到富含( S )-对映异构体的目标产物1 。拆分后回收的外消旋酸5的优异产率以及用于拆分的市售手性胺11的高回收率,以及粗产物中 ( S )-对映体的初步富集,使我们能够减少与外消旋美托咪定的经典拆分相比,手性废物几乎是 4 倍(2)。此外,排除了当前方法中使用的昂贵的Rh催化剂和手性配体,大大降低了1的制造成本。
更新日期:2024-03-20
中文翻译:

手性前体制备右美托咪定工艺研究进展:拆分-外消旋化-循环合成策略
目前的工作描述了右美托咪定 ( 1 )的新合成路线的开发,该路线作为一种多功能麻醉剂广泛应用于医院实践,且无呼吸窘迫效应。制备化合物1 的常规方法依赖于外消旋美托咪定 ( 2 ) 的拆分。然而,除了 ( S )-对映异构体的回收率较低(从 1 kg 外消旋体中回收约 0.2 kg)之外,经典拆分还受到产生富含左旋美托咪定(R -对映异构体)的手性废物的影响,该废物无法在安全且实用的方式。我们提出了右美托咪定的新前体 2-(2,3-二甲基苯基)丙酸 ( 5 ),可在初步步骤中进行拆分,而不是拆分右美托咪定1本身。然后将解析的酸 ( S )-对映体4用于合成1,并且富含 ( R )-对映体的手性废物很容易以简单、安全和可扩展的方式外消旋并再次使用。涉及( S )-酸4的进一步合成步骤包括由酰基氯16和二甲基亚砜18制备硫叶立德19 ,将化合物19转化为α-溴酮21,以及随后将其转化为盐25a - 25j。 “叠氮路线”被发现是制备氨基酮的最佳路线,提供了具有可接受的对映体纯度的产物,并且包括溴酮21与叠氮化钠反应,然后氢化所得叠氮酮30。氨基酮盐25与硫氰酸钾的马克瓦尔德环化得到右美托咪定硫代衍生物26,其脱硫以高收率得到富含( S )-对映异构体的目标产物1 。拆分后回收的外消旋酸5的优异产率以及用于拆分的市售手性胺11的高回收率,以及粗产物中 ( S )-对映体的初步富集,使我们能够减少与外消旋美托咪定的经典拆分相比,手性废物几乎是 4 倍(2)。此外,排除了当前方法中使用的昂贵的Rh催化剂和手性配体,大大降低了1的制造成本。






























 京公网安备 11010802027423号
京公网安备 11010802027423号