当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of alkynyl sulfides via base-promoted nucleophilic ring-opening of α-bromostyrene sulfonium salt
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2024-03-22 , DOI: 10.1039/d4ob00203b Junqi Zhou 1 , Ziyu Wang 1 , Hanmiao Xu 1 , Mengke Su 1 , Jian Wen 1
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2024-03-22 , DOI: 10.1039/d4ob00203b Junqi Zhou 1 , Ziyu Wang 1 , Hanmiao Xu 1 , Mengke Su 1 , Jian Wen 1
Affiliation
|
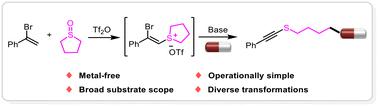
An efficient method for the synthesis of alkynyl sulfides via a C(sp3)–S bond cleavage of α-bromostyrene sulfonium salts has been developed. This base-promoted nucleophilic ring-opening pathway allows the preparation of diverse alkynyl sulfide compounds using tetramethylene sulfoxide as the sulfur source. The reaction proceeds with good functional group tolerance and could be applied to the late-stage functionalization of bioactive molecules and drugs. Furthermore, the synthetic utility of this method was demonstrated by a one-pot synthesis, scale-up reaction and further modification of various alkynyl sulfide products.
中文翻译:

碱促进α-溴苯乙烯锍盐亲核开环合成炔基硫醚
开发了一种通过α-溴苯乙烯锍盐的 C(sp 3 )-S 键断裂合成炔基硫醚的有效方法。这种碱促进的亲核开环途径允许使用四亚甲基亚砜作为硫源制备多种炔基硫醚化合物。该反应具有良好的官能团耐受性,可应用于生物活性分子和药物的后期功能化。此外,通过各种炔硫醚产品的一锅合成、放大反应和进一步修饰,证明了该方法的合成实用性。
更新日期:2024-03-22
中文翻译:

碱促进α-溴苯乙烯锍盐亲核开环合成炔基硫醚
开发了一种通过α-溴苯乙烯锍盐的 C(sp 3 )-S 键断裂合成炔基硫醚的有效方法。这种碱促进的亲核开环途径允许使用四亚甲基亚砜作为硫源制备多种炔基硫醚化合物。该反应具有良好的官能团耐受性,可应用于生物活性分子和药物的后期功能化。此外,通过各种炔硫醚产品的一锅合成、放大反应和进一步修饰,证明了该方法的合成实用性。































 京公网安备 11010802027423号
京公网安备 11010802027423号