Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Selective Hydrogenation of Azobenzene to Hydrazobenzene via Proton-Coupled Electron Transfer from a Polyoxotungstate Cluster
JACS Au ( IF 8.5 ) Pub Date : 2024-03-21 , DOI: 10.1021/jacsau.4c00127 Zhou Lu 1 , Shannon E Cooney 1 , James R McKone 2 , Ellen M Matson 1
JACS Au ( IF 8.5 ) Pub Date : 2024-03-21 , DOI: 10.1021/jacsau.4c00127 Zhou Lu 1 , Shannon E Cooney 1 , James R McKone 2 , Ellen M Matson 1
Affiliation
|
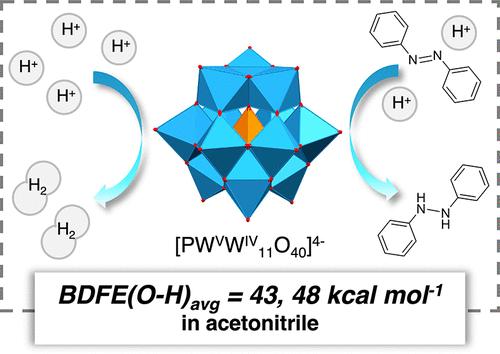
In this report, we describe proton-coupled electron transfer (PCET) reactivity at the surface of the Keggin-type polyoxotungstate cluster [nBu4N]3[PWVI12O40] (PW12) in acetonitrile. Bond dissociation free energies (BDFEs) of the O–H groups generated upon reduction of PW12 in the presence of acid are determined through the construction of a potential–pKa diagram. The surface O–H bonds are found to be weak (BDFE(O–H)avg < 48 kcal mol–1), comparable to the BDFE of H2. This is consistent with the observed formation of H2 upon addition of a suitably strong organic acid, H2NPh2+ (pKa MeCN = 5.98), to the reduced form of the cluster. The one-electron reduced form of PW12 is isolated and used in conjunction with acid to realize the stoichiometric semihydrogenation of azobenzene via PCET from the surface of the reduced cluster.
中文翻译:

通过多钨酸簇的质子耦合电子转移将偶氮苯选择性加氢为肼苯
在本报告中,我们描述了乙腈中 Keggin 型多钨酸簇 [ n Bu 4 N] 3 [PW VI 12 O 40 ] ( PW 12 ) 表面的质子耦合电子转移 (PCET) 反应性。在酸存在下还原PW 12时产生的 O-H 基团的键解离自由能 (BDFE) 通过构建势-p K a图来确定。发现表面 O-H 键很弱(BDFE(O-H) avg < 48 kcal mol –1 ),与 H 2的 BDFE 相当。这与在将适当强的有机酸H 2 NPh 2 + (p K a MeCN = 5.98)添加到簇的还原形式时观察到的H 2形成一致。分离出PW 12的单电子还原形式,并与酸结合使用,通过还原团簇表面的 PCET 实现偶氮苯的化学计量半氢化。
更新日期:2024-03-21
中文翻译:

通过多钨酸簇的质子耦合电子转移将偶氮苯选择性加氢为肼苯
在本报告中,我们描述了乙腈中 Keggin 型多钨酸簇 [ n Bu 4 N] 3 [PW VI 12 O 40 ] ( PW 12 ) 表面的质子耦合电子转移 (PCET) 反应性。在酸存在下还原PW 12时产生的 O-H 基团的键解离自由能 (BDFE) 通过构建势-p K a图来确定。发现表面 O-H 键很弱(BDFE(O-H) avg < 48 kcal mol –1 ),与 H 2的 BDFE 相当。这与在将适当强的有机酸H 2 NPh 2 + (p K a MeCN = 5.98)添加到簇的还原形式时观察到的H 2形成一致。分离出PW 12的单电子还原形式,并与酸结合使用,通过还原团簇表面的 PCET 实现偶氮苯的化学计量半氢化。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号