当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
H4octapa-Trastuzumab: Versatile Acyclic Chelate System for111In and177Lu Imaging and Therapy
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2013-08-15 , DOI: 10.1021/ja4049493 Eric W Price 1 , Brian M Zeglis , Jacqueline F Cawthray , Caterina F Ramogida , Nicholas Ramos , Jason S Lewis , Michael J Adam , Chris Orvig
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2013-08-15 , DOI: 10.1021/ja4049493 Eric W Price 1 , Brian M Zeglis , Jacqueline F Cawthray , Caterina F Ramogida , Nicholas Ramos , Jason S Lewis , Michael J Adam , Chris Orvig
Affiliation
|
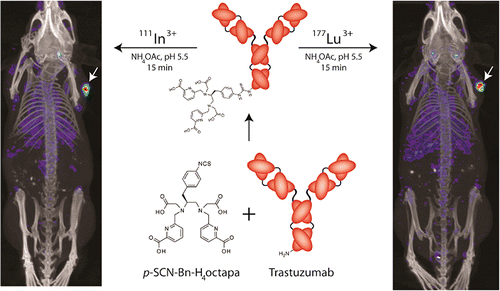
A bifunctional derivative of the versatile acyclic chelator H4octapa, p-SCN-Bn-H4octapa, has been synthesized for the first time. The chelator was conjugated to the HER2/neu-targeting antibody trastuzumab and labeled in high radiochemical purity and specific activity with the radioisotopes (111)In and (177)Lu. The in vivo behavior of the resulting radioimmunoconjugates was investigated in mice bearing ovarian cancer xenografts and compared to analogous radioimmunoconjugates employing the ubiquitous chelator 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA). The H4octapa-trastuzumab conjugates displayed faster radiolabeling kinetics with more reproducible yields under milder conditions (15 min, RT, ~94-95%) than those based on DOTA-trastuzumab (60 min, 37 °C, ~50-88%). Further, antibody integrity was better preserved in the (111)In- and (177)Lu-octapa-trastuzumab constructs, with immunoreactive fractions of 0.99 for each compared to 0.93-0.95 for (111)In- and (177)Lu-DOTA-trastuzumab. These results translated to improved in vivo biodistribution profiles and SPECT imaging results for (111)In- and (177)Lu-octapa-trastuzumab compared to (111)In- and (177)Lu-DOTA-trastuzumab, with increased tumor uptake and higher tumor-to-tissue activity ratios.
中文翻译:

H4octapa-曲妥珠单抗:用于 111In 和 177Lu 成像和治疗的多功能无环螯合物系统
首次合成了多功能无环螯合剂 H4octapa 的双功能衍生物 p-SCN-Bn-H4octapa。该螯合剂与 HER2/neu 靶向抗体曲妥珠单抗缀合,并用放射性同位素 (111)In 和 (177)Lu 以高放射化学纯度和比活性进行标记。在携带卵巢癌异种移植物的小鼠中研究了所得放射免疫缀合物的体内行为,并与使用普遍存在的螯合剂 1,4,7,10-四氮杂环十二烷-1,4,7,10-四乙酸 (DOTA) 的类似放射免疫缀合物进行了比较。与基于 DOTA-曲妥珠单抗(60 分钟,37°C,~50-88%)的那些相比,H4octapa-曲妥珠单抗缀合物在更温和的条件(15 分钟,RT,~94-95%)下表现出更快的放射性标记动力学和更高的可重复产量。此外,(111)In-和(177)Lu-octapa-曲妥珠单抗构建体中的抗体完整性得到了更好的保存,每个免疫反应分数为0.99,而(111)In-和(177)Lu-DOTA的免疫反应分数为0.93-0.95 -曲妥珠单抗。与 (111)In-和 (177)Lu-DOTA-曲妥珠单抗相比,这些结果转化为 (111)In-和 (177)Lu-octapa-曲妥珠单抗改善的体内生物分布曲线和 SPECT 成像结果,肿瘤摄取和更高的肿瘤与组织活性比率。
更新日期:2013-08-15
中文翻译:

H4octapa-曲妥珠单抗:用于 111In 和 177Lu 成像和治疗的多功能无环螯合物系统
首次合成了多功能无环螯合剂 H4octapa 的双功能衍生物 p-SCN-Bn-H4octapa。该螯合剂与 HER2/neu 靶向抗体曲妥珠单抗缀合,并用放射性同位素 (111)In 和 (177)Lu 以高放射化学纯度和比活性进行标记。在携带卵巢癌异种移植物的小鼠中研究了所得放射免疫缀合物的体内行为,并与使用普遍存在的螯合剂 1,4,7,10-四氮杂环十二烷-1,4,7,10-四乙酸 (DOTA) 的类似放射免疫缀合物进行了比较。与基于 DOTA-曲妥珠单抗(60 分钟,37°C,~50-88%)的那些相比,H4octapa-曲妥珠单抗缀合物在更温和的条件(15 分钟,RT,~94-95%)下表现出更快的放射性标记动力学和更高的可重复产量。此外,(111)In-和(177)Lu-octapa-曲妥珠单抗构建体中的抗体完整性得到了更好的保存,每个免疫反应分数为0.99,而(111)In-和(177)Lu-DOTA的免疫反应分数为0.93-0.95 -曲妥珠单抗。与 (111)In-和 (177)Lu-DOTA-曲妥珠单抗相比,这些结果转化为 (111)In-和 (177)Lu-octapa-曲妥珠单抗改善的体内生物分布曲线和 SPECT 成像结果,肿瘤摄取和更高的肿瘤与组织活性比率。






























 京公网安备 11010802027423号
京公网安备 11010802027423号