当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Functionalization of Magnetic UiO-66-NH2 with a Chiral Cu(l-proline)2 Complex as a Hybrid Asymmetric Catalyst for CO2 Conversion into Cyclic Carbonates
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2024-03-19 , DOI: 10.1021/acs.inorgchem.4c00376 Sobhan Rezayati 1 , Ali Morsali 1
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2024-03-19 , DOI: 10.1021/acs.inorgchem.4c00376 Sobhan Rezayati 1 , Ali Morsali 1
Affiliation
|
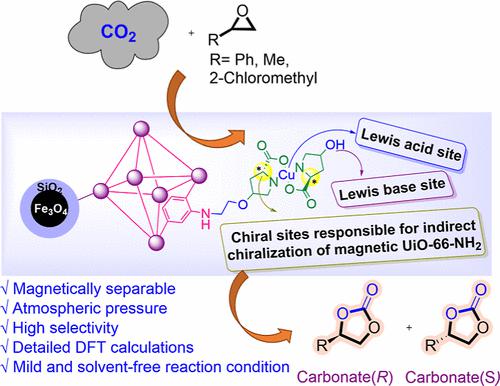
In this study, a chiral [Cu(l-proline)2] complex-modified Fe3O4@SiO2@UiO-66-NH2(Zr) metal–organic framework [Fe3O4@SiO2@UiO-66-NH-Cu(l-proline)2] via multifunctionalization strategies was designed and synthesized. One simple approach to chiralize an achiral MOF-structure that cannot be directly chiralized using a chiral secondary agent like 4-hydroxy-l-proline. Therefore, this chiral catalyst was synthesized with a simple and multistep method. Accordingly, Fe3O4@SiO2@UiO-66-NH2 has been synthesized via Fe3O4 modification with tetraethyl orthosilicate and subsequently with ZrCl4 and 2-aminoterephthalic acid. The presence of the silica layer helps to stabilize the Fe3O4 core, while the bonding between Zr4+ and the –OH groups in the silica layer promotes the development of Zr-MOFs on the Fe3O4 surface, and then the surfaces of the synthesized magnetic MOFs composite are functionalized with 1,2-dichloroethane and Cu(II) complex with 4-hydroxy-l-proline, [Cu(l-proline)2] to afford the magnetically chiral nanocatalyst. Multiple techniques were employed to characterize this magnetically chiral nanocatalyst such as Fourier transform infrared (FT-IR), X-ray photoelectron spectroscopy (XPS), field-emission scanning electron microscopy (FE-SEM), transmission electron microscopy (TEM), energy-dispersive X-ray spectrometry (EDX), powder X-ray diffraction (PXRD), circular dichroism (CD), inductively coupled plasma (ICP), thermogravimetric analysis (TGA), vibrating-sample magnetometry (VSM), and Brunauer–Emmett–Teller (BET) analyses. Moreover, a magnetically chiral nanocatalyst shows the asymmetric CO2 fixation reaction under solvent-free conditions at 80 °C and in ethanol under reflux conditions with up to 99 and 98% ee, respectively. Furthermore, the reaction mechanism was illustrated concerning the total energy of the reactant, intermediates and product, and the structural parameters were analyzed.
中文翻译:

磁性 UiO-66-NH2 与手性 Cu(l-脯氨酸)2 配合物的功能化作为 CO2 转化为环状碳酸酯的混合不对称催化剂
本研究中,手性[Cu( l-脯氨酸) 2 ]络合物修饰Fe 3 O 4 @SiO 2 @UiO-66-NH 2 (Zr)金属有机骨架[Fe 3 O 4 @SiO 2 @UiO-通过多功能化策略设计并合成了66-NH-Cu( l-脯氨酸) 2 ]。一种手性化非手性 MOF 结构的简单方法,该结构不能使用 4-羟基-l-脯氨酸等手性辅助剂直接手性化。因此,该手性催化剂是通过简单的多步方法合成的。因此,通过用原硅酸四乙酯、随后用ZrCl 4和2-氨基对苯二甲酸对Fe 3 O 4进行改性,合成了Fe 3 O 4 @SiO 2 @UiO-66-NH 2 。二氧化硅层的存在有助于稳定Fe 3 O 4核,而二氧化硅层中Zr 4+和-OH基团之间的键合促进了Zr-MOFs在Fe 3 O 4表面的发展,然后合成的磁性MOF复合材料的表面用1,2-二氯乙烷和Cu(II)与4-羟基-l-脯氨酸的络合物[Cu( l-脯氨酸) 2 ]功能化,以提供磁手性纳米催化剂。 采用多种技术来表征这种磁手性纳米催化剂,例如傅里叶变换红外(FT-IR)、X射线光电子能谱(XPS)、场发射扫描电子显微镜(FE-SEM)、透射电子显微镜(TEM)、能量-色散 X 射线光谱 (EDX)、粉末 X 射线衍射 (PXRD)、圆二色性 (CD)、电感耦合等离子体 (ICP)、热重分析 (TGA)、振动样品磁力测定 (VSM) 和 Brunauer-Emmett –Teller (BET) 分析。此外,磁手性纳米催化剂在80℃无溶剂条件下和在回流条件下乙醇中分别表现出高达99%和98% ee的不对称CO 2固定反应。此外,从反应物、中间体和产物的总能量阐述了反应机理,并对结构参数进行了分析。
更新日期:2024-03-19
中文翻译:

磁性 UiO-66-NH2 与手性 Cu(l-脯氨酸)2 配合物的功能化作为 CO2 转化为环状碳酸酯的混合不对称催化剂
本研究中,手性[Cu( l-脯氨酸) 2 ]络合物修饰Fe 3 O 4 @SiO 2 @UiO-66-NH 2 (Zr)金属有机骨架[Fe 3 O 4 @SiO 2 @UiO-通过多功能化策略设计并合成了66-NH-Cu( l-脯氨酸) 2 ]。一种手性化非手性 MOF 结构的简单方法,该结构不能使用 4-羟基-l-脯氨酸等手性辅助剂直接手性化。因此,该手性催化剂是通过简单的多步方法合成的。因此,通过用原硅酸四乙酯、随后用ZrCl 4和2-氨基对苯二甲酸对Fe 3 O 4进行改性,合成了Fe 3 O 4 @SiO 2 @UiO-66-NH 2 。二氧化硅层的存在有助于稳定Fe 3 O 4核,而二氧化硅层中Zr 4+和-OH基团之间的键合促进了Zr-MOFs在Fe 3 O 4表面的发展,然后合成的磁性MOF复合材料的表面用1,2-二氯乙烷和Cu(II)与4-羟基-l-脯氨酸的络合物[Cu( l-脯氨酸) 2 ]功能化,以提供磁手性纳米催化剂。 采用多种技术来表征这种磁手性纳米催化剂,例如傅里叶变换红外(FT-IR)、X射线光电子能谱(XPS)、场发射扫描电子显微镜(FE-SEM)、透射电子显微镜(TEM)、能量-色散 X 射线光谱 (EDX)、粉末 X 射线衍射 (PXRD)、圆二色性 (CD)、电感耦合等离子体 (ICP)、热重分析 (TGA)、振动样品磁力测定 (VSM) 和 Brunauer-Emmett –Teller (BET) 分析。此外,磁手性纳米催化剂在80℃无溶剂条件下和在回流条件下乙醇中分别表现出高达99%和98% ee的不对称CO 2固定反应。此外,从反应物、中间体和产物的总能量阐述了反应机理,并对结构参数进行了分析。

































 京公网安备 11010802027423号
京公网安备 11010802027423号