当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Diastereoselective (3 + 3)-Annulations of Trisubstituted Michael Acceptors for Access to Polyfunctional Cyclohexanones
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2024-03-19 , DOI: 10.1021/acs.joc.4c00157 Katelyn M Kitzinger 1 , Mitchell T Giordano 1 , Pedro de Jesús Cruz 1 , Jeffrey S Johnson 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2024-03-19 , DOI: 10.1021/acs.joc.4c00157 Katelyn M Kitzinger 1 , Mitchell T Giordano 1 , Pedro de Jesús Cruz 1 , Jeffrey S Johnson 1
Affiliation
|
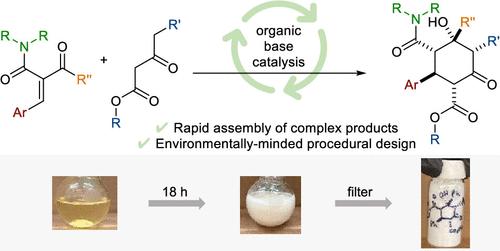
Michael-aldol domino reactions are powerful tools for rapidly assembling carbocyclic scaffolds. We herein disclose a base-catalyzed Michael-aldol domino reaction of trisubstituted Michael acceptors with β-keto ester nucleophiles. The cyclohexanone products are obtained in excellent diastereoselectivity (up to >20:1 dr) and good yields (up to 84%). An attractive practical consideration is that pure products are isolated directly via filtration of the unpurified reaction mixtures. Further functionalization of the cyclohexanones is achieved without perturbation of stereocenters installed through the preceding annulation.
中文翻译:

用于获得多官能环己酮的三取代迈克尔受体的非对映选择性 (3 + 3)-环化
迈克尔-羟醛多米诺反应是快速组装碳环支架的有力工具。我们在此公开了三取代的迈克尔受体与β-酮酯亲核试剂的碱催化的迈克尔-羟醛多米诺反应。环己酮产品具有优异的非对映选择性(高达 >20:1 dr)和良好的产率(高达 84%)。一个有吸引力的实际考虑是通过过滤未纯化的反应混合物直接分离纯产物。环己酮的进一步官能化是在不扰动通过先前成环安装的立构中心的情况下实现的。
更新日期:2024-03-19
中文翻译:

用于获得多官能环己酮的三取代迈克尔受体的非对映选择性 (3 + 3)-环化
迈克尔-羟醛多米诺反应是快速组装碳环支架的有力工具。我们在此公开了三取代的迈克尔受体与β-酮酯亲核试剂的碱催化的迈克尔-羟醛多米诺反应。环己酮产品具有优异的非对映选择性(高达 >20:1 dr)和良好的产率(高达 84%)。一个有吸引力的实际考虑是通过过滤未纯化的反应混合物直接分离纯产物。环己酮的进一步官能化是在不扰动通过先前成环安装的立构中心的情况下实现的。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号