当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Chiral π-Conjugated Double Helical Aminyl Diradical with the Triplet Ground State
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-03-19 , DOI: 10.1021/jacs.4c02057 Haoxin Guo 1 , Joshua B Lovell 2 , Chan Shu 1 , Maren Pink 3 , Martha Morton 1 , Suchada Rajca 1 , Andrzej Rajca 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-03-19 , DOI: 10.1021/jacs.4c02057 Haoxin Guo 1 , Joshua B Lovell 2 , Chan Shu 1 , Maren Pink 3 , Martha Morton 1 , Suchada Rajca 1 , Andrzej Rajca 1
Affiliation
|
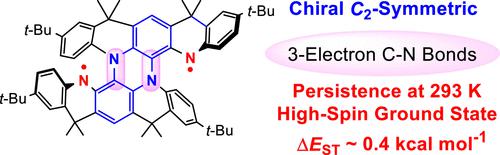
We report a neutral high-spin diradical of chiral C2-symmetric bis[5]diazahelicene with ΔEST ≈ 0.4 kcal mol–1, as determined by EPR spectroscopy/SQUID magnetometry. The diradical is the most persistent among all high-spin aminyl radicals reported to date by a factor of 20, with a half-life of up to 6 days in 2-MeTHF at room temperature. Its triplet ground state and excellent persistence may be associated with the unique spin density distribution within the dihydrophenazine moiety, which characterizes two effective 3-electron C–N bonds analogous to the N–O bond of a nitroxide radical. The enantiomerically enriched (ee ≥ 94%) (MM)- and (PP)-enantiomers of the precursors to the diradicals are obtained by either preparative chiral supercritical fluid chromatography or resolution via functionalization with the chiral auxiliary of the C2-symmetric racemic tetraamine. The barrier for the racemization of the solid tetraamine is ΔG‡ = 43 ± 0.01 kcal mol–1 in the 483–523 K range. The experimentally estimated lower limit of the barrier for the racemization of a diradical, ΔG‡ ≥ 26 kcal mol–1 in 2-MeTHF at 293 K, is comparable to the DFT-determined barrier of ΔG‡ = 31 kcal mol–1 in the gas phase at 298 K. While the enantiomerically pure tetraamine displays strong chiroptical properties, with anisotropy factor |g| = |Δε|/ε = 0.036 at 376 nm, |g| ≈ 0.005 at 548 nm of the high-spin diradical is comparable to that recently reported triplet ground-state diradical dication. Notably, the radical anion intermediate in the generation of diradical exhibits a large SOMO-HOMO inversion, SHI = 35 kcal mol–1.
中文翻译:

具有三重基态的手性 π-共轭双螺旋氨基双自由基
我们报告了手性C 2对称双[5]二氮杂环烯的中性高自旋双自由基,其 Δ E ST ≈ 0.4 kcal mol –1 ,由 EPR 光谱/SQUID 磁力测定法测定。双自由基是迄今为止报道的所有高自旋氨基自由基中最持久的,其持久性是其 20 倍,室温下在 2-MeTHF 中的半衰期长达 6 天。其三线态基态和优异的持久性可能与二氢吩嗪部分内独特的自旋密度分布有关,该分布表征了两个有效的3电子C-N键,类似于硝基氧自由基的N-O键。双自由基前体的对映体富集 (ee ≥ 94%) ( MM )-和 ( PP )-对映体可通过制备型手性超临界流体色谱法或通过使用C 2对称外消旋四胺的手性助剂进行官能化来获得。在 483–523 K 范围内,固体四胺外消旋化的势垒为 Δ G ‡ = 43 ± 0.01 kcal mol –1 。实验估计 293 K 2-MeTHF 中双自由基外消旋化势垒下限 Δ G ‡ ≥ 26 kcal mol –1与 DFT 确定的势垒 Δ G ‡ = 31 kcal mol –1相当在 298 K 的气相中。而对映体纯的四胺表现出很强的手性光学特性,具有各向异性因子 |克| = |Δε|/ε = 0.036(376 nm),|克|高自旋双自由基在 548 nm 处的 ≈ 0.005 与最近报道的三重基态双自由基双自由基相当。 值得注意的是,双自由基生成中的自由基阴离子中间体表现出大的 SOMO-HOMO 反转,SHI = 35 kcal mol –1 。
更新日期:2024-03-19
中文翻译:

具有三重基态的手性 π-共轭双螺旋氨基双自由基
我们报告了手性C 2对称双[5]二氮杂环烯的中性高自旋双自由基,其 Δ E ST ≈ 0.4 kcal mol –1 ,由 EPR 光谱/SQUID 磁力测定法测定。双自由基是迄今为止报道的所有高自旋氨基自由基中最持久的,其持久性是其 20 倍,室温下在 2-MeTHF 中的半衰期长达 6 天。其三线态基态和优异的持久性可能与二氢吩嗪部分内独特的自旋密度分布有关,该分布表征了两个有效的3电子C-N键,类似于硝基氧自由基的N-O键。双自由基前体的对映体富集 (ee ≥ 94%) ( MM )-和 ( PP )-对映体可通过制备型手性超临界流体色谱法或通过使用C 2对称外消旋四胺的手性助剂进行官能化来获得。在 483–523 K 范围内,固体四胺外消旋化的势垒为 Δ G ‡ = 43 ± 0.01 kcal mol –1 。实验估计 293 K 2-MeTHF 中双自由基外消旋化势垒下限 Δ G ‡ ≥ 26 kcal mol –1与 DFT 确定的势垒 Δ G ‡ = 31 kcal mol –1相当在 298 K 的气相中。而对映体纯的四胺表现出很强的手性光学特性,具有各向异性因子 |克| = |Δε|/ε = 0.036(376 nm),|克|高自旋双自由基在 548 nm 处的 ≈ 0.005 与最近报道的三重基态双自由基双自由基相当。 值得注意的是,双自由基生成中的自由基阴离子中间体表现出大的 SOMO-HOMO 反转,SHI = 35 kcal mol –1 。































 京公网安备 11010802027423号
京公网安备 11010802027423号