Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
没食子酸改性聚乙烯亚胺-聚丙烯碳酸酯-聚乙烯亚胺纳米粒子:合成、表征和抗牙周炎评估
ACS Omega ( IF 3.7 ) Pub Date : 2024-03-15 , DOI: 10.1021/acsomega.4c00261 Zunxuan Xie 1 , Boyang Gao 1 , Jinyao Liu 1 , Jiaming He 1 , Yuyan Liu 1 , Fengxiang Gao 2
Affiliation
|
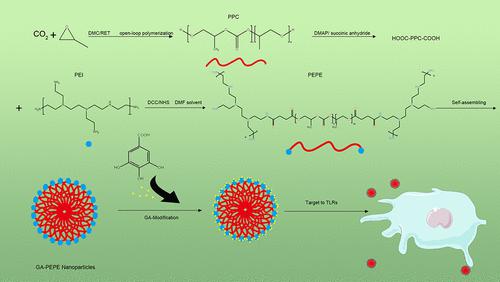
该研究的目的是开发新型没食子酸(GA)修饰的聚乙烯亚胺(PEI)-聚碳酸亚丙酯(PPC)-PEI(PEPE)两亲性纳米颗粒,并全面评估其作为靶向Toll样受体的抗牙周炎纳米颗粒的特性。 TLR)。第一步是使用分子对接技术评估 GA 与牙周炎核心触发受体 TLR2 和 TLR4/MD2 的结合潜力。随后,我们对合成的 PEPE 纳米粒子进行了核磁共振、透射电子显微镜和动态光散射分析。最后一步,我们研究了 GA-PEPE 纳米粒子的合成结果和体外抗牙周炎特性。研究表明,GA 具有靶向结合 TLR2 和 TLR4/MD2 复合物的潜力。此外,我们成功开发了91.19 nm带正电的PEPE纳米颗粒。光谱分析表明GA修饰的PEPE成功合成。此外,CCK8 结果表明,GA 修饰显着降低了 PEPE 的生物毒性。体外抗牙周炎特性评估表明,6.25 μM GA-PEPE 纳米颗粒显着降低促炎因子 TNF-α、IL-1β 和 IL-6 的表达。 GA-PEPE 纳米颗粒具有靶向 TLR 结合能力,具有优异的生物相容性和抗牙周炎特性。 GA-PEPE纳米颗粒将为抗牙周炎纳米颗粒的开发提供高度创新的投入。

"点击查看英文标题和摘要"





















































 京公网安备 11010802027423号
京公网安备 11010802027423号