当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Total Syntheses of Phorbol and 11 Tigliane Diterpenoids and Their Evaluation as HIV Latency-Reversing Agents
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-03-14 , DOI: 10.1021/jacs.4c01589 Ayumu Watanabe 1 , Masanori Nagatomo 1 , Akira Hirose 1 , Yuto Hikone 1 , Naoki Kishimoto 2 , Satoshi Miura 2 , Tae Yasutake 2 , Towa Abe 2 , Shogo Misumi 2 , Masayuki Inoue 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-03-14 , DOI: 10.1021/jacs.4c01589 Ayumu Watanabe 1 , Masanori Nagatomo 1 , Akira Hirose 1 , Yuto Hikone 1 , Naoki Kishimoto 2 , Satoshi Miura 2 , Tae Yasutake 2 , Towa Abe 2 , Shogo Misumi 2 , Masayuki Inoue 1
Affiliation
|
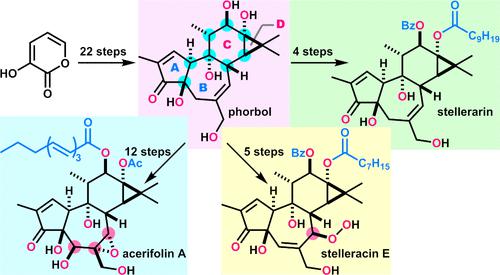
Tigliane diterpenoids possess exceptionally complex structures comprising common 5/7/6/3-membered ABCD-rings and disparate oxygen functionalities. While tiglianes display a wide range of biological activities, compounds with HIV latency-reversing activity can eliminate viral reservoirs, thereby serving as promising leads for new anti-HIV agents. Herein, we report collective total syntheses of phorbol (13) and 11 tiglianes 14–24 with various acylation patterns and oxidation states, and their evaluation as HIV latency-reversing agents. The syntheses were strategically divided into five stages to increase the structural complexity. First, our previously established sequence enabled the expeditious preparation of ABC-tricycle 9 in 15 steps. Second, hydroxylation of 9 and ring-contractive D-ring formation furnished phorbol (13). Third, site-selective attachment of two acyl groups to 13 produced four phorbol diesters 14–17. Fourth, the oxygen functionalities were regio- and stereoselectively installed to yield five tiglianes 18–22. Fifth, further oxidation to the most densely oxygenated acerifolin A (23) and tigilanol tiglate (24) was realized through organizing a 3D shape of the B-ring. Assessment of the HIV latency-reversing activities of the 12 tiglianes revealed seven tiglianes (14–17 and 22–24) with 20- to 300-fold improved efficacy compared with prostratin (12), a representative latency-reversing agent. Therefore, the robust synthetic routes to a variety of tiglianes with promising activities devised in this study provide opportunities for advancing HIV eradication strategies.
中文翻译:

佛波醇和 11 种 Tigliane 二萜的全合成及其作为 HIV 潜伏期逆转剂的评价
Tigliane 二萜类化合物具有极其复杂的结构,包括常见的 5/7/6/3 元 ABCD 环和不同的氧官能团。虽然提利亚内酯显示出广泛的生物活性,但具有 HIV 潜伏期逆转活性的化合物可以消除病毒储存库,从而成为新型抗 HIV 药物的有希望的先导化合物。在此,我们报告了具有各种酰化模式和氧化态的佛波醇 ( 13 ) 和 11 种提利亚烷14 – 24的集体全合成,以及它们作为 HIV 潜伏期逆转剂的评估。合成被战略性地分为五个阶段以增加结构的复杂性。首先,我们之前建立的序列可以通过 15 个步骤快速制备 ABC-三轮车9 。其次, 9的羟基化和环收缩 D 环的形成提供了佛波醇 ( 13 )。第三,两个酰基与13的位点选择性连接产生了四种佛波二酯14 – 17 。第四,氧官能团被区域和立体选择性地安装以产生五个提利亚内酯18-22 。第五,通过组织B环的3D形状,进一步氧化为最密集氧化的acerifolin A ( 23 )和tigilanol tiglate ( 24 )。对 12 种 tiglianes 的 HIV 潜伏期逆转活性的评估显示,与代表性潜伏期逆转剂 Prostratin ( 12 ) 相比,7 种 tiglianes( 14 – 17和22 – 24 )的疗效提高了 20 至 300 倍。 因此,本研究设计的各种具有前景活性的替利亚内酯的稳健合成路线为推进艾滋病毒根除策略提供了机会。
更新日期:2024-03-14
中文翻译:

佛波醇和 11 种 Tigliane 二萜的全合成及其作为 HIV 潜伏期逆转剂的评价
Tigliane 二萜类化合物具有极其复杂的结构,包括常见的 5/7/6/3 元 ABCD 环和不同的氧官能团。虽然提利亚内酯显示出广泛的生物活性,但具有 HIV 潜伏期逆转活性的化合物可以消除病毒储存库,从而成为新型抗 HIV 药物的有希望的先导化合物。在此,我们报告了具有各种酰化模式和氧化态的佛波醇 ( 13 ) 和 11 种提利亚烷14 – 24的集体全合成,以及它们作为 HIV 潜伏期逆转剂的评估。合成被战略性地分为五个阶段以增加结构的复杂性。首先,我们之前建立的序列可以通过 15 个步骤快速制备 ABC-三轮车9 。其次, 9的羟基化和环收缩 D 环的形成提供了佛波醇 ( 13 )。第三,两个酰基与13的位点选择性连接产生了四种佛波二酯14 – 17 。第四,氧官能团被区域和立体选择性地安装以产生五个提利亚内酯18-22 。第五,通过组织B环的3D形状,进一步氧化为最密集氧化的acerifolin A ( 23 )和tigilanol tiglate ( 24 )。对 12 种 tiglianes 的 HIV 潜伏期逆转活性的评估显示,与代表性潜伏期逆转剂 Prostratin ( 12 ) 相比,7 种 tiglianes( 14 – 17和22 – 24 )的疗效提高了 20 至 300 倍。 因此,本研究设计的各种具有前景活性的替利亚内酯的稳健合成路线为推进艾滋病毒根除策略提供了机会。














































 京公网安备 11010802027423号
京公网安备 11010802027423号